Heart Failure, Hope, and the Hurdles Ahead for Xenotransplantation
Genetically altered pig hearts represent a major step forward, but replacing the myriad functions of a human heart? Time will tell.

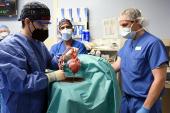
Last month, 57-year-old David Bennett became the first patient in the world to receive a genetically modified pig heart. At the time, he was critically ill with end-stage heart failure and had been hospitalized at the University of Maryland Medical Center (UMMC) since October after presenting in cardiogenic shock and being placed on heart-lung bypass machine as a desperate measure. The prospects were grim, because he was not a candidate for a ventricular assist device or heart transplantation.
Unfortunately, for an advanced heart failure and transplant team, these extremely critical situations are not particularly rare. Like most cardiologists, I’ve taken care of many patients with heart failure who, despite our best efforts, died waiting for a heart transplantation. The numbers are stark. Approximately 6.5 million Americans have heart failure, which contributes to approximately 287,000 deaths a year. Yet mainly due to shortage of donor hearts, only ~3,500 heart transplants are performed in the US each year. That means, the vast majority of patients with advance heart failure, who would otherwise benefit from lifesaving heart transplants, will die each year. In fact, 17 people die each day waiting for an organ transplant. If we could replace diseased organs with pig organs, we may be able to overcome this key bottleneck.
SEE ALSO: Realistic Expectations Emerge After Initial Excitement Over Xenotransplant
The surgery led by Dr. Bartley Griffith and Dr. Muhammad Mohiuddin, co-directors of the Cardiac Xenotransplantation Program at the University of Maryland School of Medicine, represents a major milestone, though one of many in the decades of long progress in xenotransplantation science.
Pioneering studies by Dr. Mohiuddin and others have documented hyperacute rejection mediated by preformed antibodies against certain sugar residues on pig tissues, vascular thrombosis due to suboptimal coagulation regulation, and donor cell injury from aberrant immune complement activation. Based on these early findings, the donor pig was genetically engineered to be “humanized” to specifically overcome these barriers.
Additionally, Dr. Mohiuddin first demonstrated that blocking the immune co-stimulatory molecule CD40, along with a traditional immunosuppression regimen, could prolong graft survival to almost 3 years when the genetically engineered pig hearts were transplanted into baboons. Lastly, because these studies showed abnormal pig heart growth, resulting in overly stiff and thick heart tissue, the donor pig heart was genetically engineered to lack the growth hormone receptor. Still, there are numerous unknowns, including the theoretical concern for transmission of ancient retroviruses in the pig genome.
Thus far, the patient is doing remarkably well. I think it’s safe to say the dreaded hyperacute rejection barrier of xenotransplantation has been broken. But enormous hurdles remain, including acute or chronic rejection, opportunistic infections, thickening and stiffening of the heart, and severe deconditioning due to his long-standing heart failure. While ultimate outcome for this patient is unknown and everyone remains guarded, with each passing day, I become increasingly optimistic that this may be the beginning of what could only be imagined before.
No medical technology is perfect. Even recipients of human donor hearts must take a lifetime of immunosuppressants to prevent organ rejection, and live with concerns for transplant vasculopathy, secondary infections, and increased risk of malignancies. Also, comparisons have been raised regarding emerging technologies like left ventricular assist devices and total artificial hearts. While advances in these technologies have been breathtaking, it is not clear whether they can replace every function of the heart, especially in light of the emerging understanding of the heart’s myriad endocrine functions.
These technologies often lead to muscle wasting in the patient before transplant, which can add extreme challenges to the recovery of the patient posttransplant. Some of these patients never return to their former independent lives. I can’t help but wonder whether these purely mechanical devices will follow the path taken by hemodialysis, which while lifesaving remains a suboptimal replacement for a kidney despite refinements made more than six decades after its introduction.
It’s also unknown whether a genetically modified pig heart will function in every way like a human heart. So far, this heart has exceeded our expectations in terms of its function and acceptability by the body. Whether it will fulfill all of the endocrine functions of the human heart—including its role in the synthesis of a number of cytokines, natriuretic peptides, growth factors and exosomes—remains an important area for further study.
Looking into the future, one can easily imagine numerous scenarios in which genomic engineering may result in “immunologically silent” donor pig organs that obviate the need for immunosuppressive therapy. Perhaps the biggest aftereffect of this landmark surgery may be that there will now by heightened urgency at the US Food and Drug Administration to provide clear regulatory guidance for xenotransplantation, stimulating sorely needed investments in this field. Finally, if xenotransplantation ultimately proves to be a viable path technically, there will be vigorous conversations about the costs and resource allocation. We welcome them.
In a news conference announcing this surgery, Dr. Griffith mentioned that our patient wanted two things: one, to live, and two, for us to learn from his experience. As we navigate this uncharted territory, we will honor the patient’s courage by sharing our experience and lessons with the rest of the world.
Finally, as a scientist, I know that only a well-controlled clinical study with multiple patients can demonstrate durability and reproducibility of xenotransplantation; however, as a physician, I am reminded that this patient has a name. Mr. David Bennett has gotten one last shot at life with this extraordinary surgery and taken a big first step for the future of this field.
Off Script is a first-person blog written by leading voices in the field of cardiology. It does not reflect the editorial position of TCTMD.
Charles (Chaz) Hong, MD, PhD, is the Melvin Sharoky, MD, Professor of Medicine and Director of Cardiology Research at the…
Read Full Bio


Comments