REDUCE LAP-HF II: Atrial Shunt Device Comes Up Short in HFpEF
The shunt, designed to relieve pulmonary capillary wedge pressure, was no better than a sham procedure, researchers said.

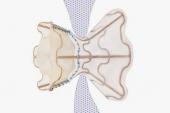
Placement of an atrial shunt device intended to reduce pulmonary capillary wedge pressure (PCWP) during exercise in patients with heart failure and preserved ejection fraction (HFpEF) does not reduce HF events or improve health status, results from the randomized, sham-controlled REDUCE LAP-HF II trial show.
Rates of the primary endpoint—a hierarchical composite of cardiovascular death or nonfatal ischemic stroke at 12 months, total HF events up to 24 months, and change in Kansas City Cardiomyopathy Questionnaire overall summary score at 12 months—were no different between patients who received the device via femoral venous access and transseptal puncture and those who had a sham procedure.
Sanjiv Shah, MD (Northwestern University Feinberg School of Medicine, Chicago, IL), presented the REDUCE LAP-HF II findings today at the inaugural Technology and Heart Failure Therapeutics (THT) 2022 meeting in New York. They were published simultaneously in the Lancet.
“The placement of the atrial shunt device did not reduce the overall rate of heart failure events, or improve health status in these patients,” Shah said, stressing that HFpEF represents a heterogeneous group of patients in whom, as drug trials have suggested, a one-size-fits-all approach may not be appropriate.
Still, these results, from the largest randomized, sham-controlled device trial to date in this patient population, are disappointing after the more-promising results of the feasibility trial REDUCE LAP-HF I. As reported by TCTMD, REDUCE LAP-HF I results were first presented at the American Heart Association 2017 Scientific Sessions, showing that this specific atrial shunt device (Corvia) decreased PCWP during exercise in HF patients with preserved or with moderately impaired ejection fraction. Those findings and others have spurred a flurry of innovation into devices intended to decompress an overloaded left atrium by helping shunt blood to the right. There are at least five different devices currently under study, three of which—including Corvia—have CE Mark in Europe.
Primary Results
REDUCE LAP-HF II enrolled 1,072 patients, of whom 626 were ultimately randomized to either the atrial shunt device or a sham procedure. All patients had to have an ejection fraction of 40% or higher and exercise PCWP ≥ 25 mmHg. The trial was conducted at 89 sites in the US, Canada, Europe, Australia, and Japan. Of note—and rare for a cardiovascular clinical trial—62% of clinical trial participants were women.
At 12 months, there was no significant difference in the cumulative rate of cardiovascular death or stroke between the device and sham groups, although it was numerically lower in the sham-treated patients. At 24 months, there was no difference in cumulative rates of heart failure events, with an analysis of individual endpoints showing no meaningful differences between groups. Results for a composite safety endpoint were not statistically different, but major cardiac events were more common in the atrial shunt group compared with the sham control: 4% versus 1% (P = 0.025).
Changes in Kansas City Cardiomyopathy Questionnaire score were no different between groups.
No late issues were seen with shunt occlusion or stenosis at 1 year (an issue seen in an early feasibility study with a different interatrial shunt device), Shah confirmed during the Q&A that followed his presentation, noting that patency was 100% at 12 months in REDUCE LAP-HF II.
’Responder Group‘ Signal
The only signal of benefit to emerge in REDUCE LAP-HF II was in a prespecified subgroup of patients who did not have evidence of pulmonary vascular disease (PVD) on an invasive exercise test, which Shah characterized as a “large potential responder group.”
Delving deeper into this subset during a separate THT 2022 presentation, study co-investigator Barry Borlaug, MD (Mayo Clinic, Rochester, MN), reviewed the biological reasons why the presence of latent PVD in HF patients with preserved or midrange EF (HFmrEF) might be harmed by atrial shunt implantation, while the opposite is true of patients without latent PVD, defined by exercise pulmonary vascular resistance lower than 1.8 Wood units.
And indeed, said Borlaug, post hoc analysis of trial participants according to presence or absence of latent PVD suggests that latent disease plays an important role in HFpEF/HFmrEF, and that invasive exercise phenotyping of patients prior to shunt placement is important for devices and procedures that rely on atrial shunt creation.
In discussions following the presentations today, panelist Horst Sievert, MD (CardioVascular Center Frankfurt CVC, Germany), who participated in some of the earliest studies in this space, said that he wasn’t previously aware of latent pulmonary vascular disease as a subgroup of concern with this therapy. He questioned whether measuring pulmonary vascular resistance would really be feasible in clinical practice, pointing out that invasive measurements of pulmonary vascular resistance during exercise is “not easy” and leads to “lots of variability.”
In response, Shah said that the trialists had faced similar criticisms with their design, but had proven in an international trial that more than 600 patients could successfully undergo invasive exercise hemodynamic screening. In the aftermath, he said, colleagues have approached him at meetings to say that performing these tests in their patients with shortness of breath has “finally” given them answers for patients for whom other tests came up empty.
“So I think it’s very feasible, once you start doing it,” said Shah.
Speaking with TCTMD, Carlos Santos-Gallego, MD (Icahn School of Medicine at Mount Sinai, New York, NY), who caught the trial’s live presentation, said that the overwhelming impression he’s seen from meeting attendees in New York is amazement and admiration for the fact that these invasive exercise tests were done.
“Of course, people are disappointed by the results, because we always hope for a positive clinical trial so we'll be able to help our patients, and also we’d expected some benefit,” he said. “But the global comment by literally everyone I've met is that it's amazing they were able to recruit more than 600 patients and perform invasive exercise tests, and it wasn't that they just did exercise tests at five or six academic institutions. No, they did it in more than 80 hospitals around the world, so that is incredible.”
This may help to establish invasive pulmonary vascular resistance testing as a “gold standard” for HFpEF diagnostics, he added. With time, this testing may become more widely available and would help refine the diagnosis of patients regularly hospitalized for shortness of breath for whom other heart failure diagnostics haven’t proved fruitful, although “we are still not close to that,” Santos-Gallego acknowledged.
In an accompanying editorial, Navin K. Kapur, MD (Tufts Medical Center, Boston, MA), and colleagues also identify a silver lining in the fact that both groups showed improvements in health status, “an observation that almost certainly limits the potential to see a treatment effect,” they write. The study also helps to answer pertinent questions around who might benefit, and who might not. “In answering these questions, we can hopefully determine whether the future armamentarium of heart failure therapies should include interatrial shunts and identify the correct population likely to benefit from this innovative approach.”
The fact that the overall trial results were negative, however, calls into question the relevance of the signal. As REDUCE LAP-HF II co-investigator Philipp Lurz, MD, PhD (University of Leipzig, German), observed to TCTMD: "Post hoc analysis and identification of responders in a negative trial is very difficult. The concept is intriguing, but it would need to be proven, and I wonder whether this will be done in future trials."
Other trials are underway: tomorrow at THT 2022, JoAnn Lindenfeld, MD (Vanderbilt University Medical Center, Nashville, TN), will present results from the roll-in cohort of RELIEVE-HF, taking place at 120 sites in the US, Canada, Europe, Israel, Australia, and New Zealand using the V-Wave interatrial shunt (Ventura).
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Shah SJ, Borlaug BA, Chung ES, et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial. Lancet. 2022:Epub ahead of print.
Disclosures
- Shah reports grant/research support from Corvia and Pfizer, consulting fees/honoraria from AstraZeneca, Aria CV, Axon Therapies, Bayer, Boehringer-Ingelheim, Boston Scientific, Edwards Lifesciences, Eidos, Imara, Ionis, Merck, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Roche, Shifamed, and Tenaya, and royalty income from UpToDate and Springer.
- Borlaug reports grant support/research contracts from Axon, AstraZeneca, Corvia, Medtronic, GlaxoSmithKline, and Tenax and consulting fees/honoraria/speakers bureau payments from Amgen, Aria, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Novo Nordisk, and VADovations.
- Sievert reports institutional support from 4tech Cardio, Abbott, Ablative Solutions, Ancora Heart, Append Medical, Axon, Bavaria Medizin Technologie GmbH, Bioventrix, Boston Scientific, Carag, Cardiac Dimensions, Cardiac Success, Cardimed, Celonova, Contego, CVRx, Dinova, Edwards, Endomatic; Hemoteq, Hangzhou Nuomao Medtech, Holistick Medical, K2, Lifetech, Mokita, Occlutech, Recor, Renal Guard, Trisol, Vascular Dynamics, Vectorious Medtech, Venus, Venock, and Vivasure Medical.
- Lurz reports institutional payments in the form of grant support/research contracts as well as consultant fee/honoraria/speakers bureau from Abbott Vascular, Edwards Lifesciences, Occlutech, ReCor and Medtronic, and personal consultant fee/honoraria/speakers bureau payments from ReCor and Innoventrics.
- Kapur reports no competing interests.


Comments