Signs of Cardiac Damage Even in Younger, Nonhospitalized COVID-19 Patients
A series of middle-aged patients followed more than 2 months after diagnosis showed that most had abnormal results on CMR.
There are more signs that COVID-19 may cause damage to the heart that lasts beyond the acute phase, based on an imaging study conducted at a single center in Germany. Despite the fact that 67% of the patients who volunteered for the study never required hospitalization, 78% had abnormal cardiac magnetic resonance (CMR) findings 2 to 3 months after testing positive for the virus.
“These were not the patients that had problems or any cardiac symptoms at all,” lead author Valentina Puntmann, MD, PhD (University Hospital Frankfurt, Germany), told TCTMD, explaining that anyone with a positive COVID-19 test result had been invited to participate through the hospital’s testing clinic. “Quite a few of them did feel shortness of breath but they didn't think it came from the heart, just that they hadn't fully recovered—something was not right.”
After being swamped with severely ill patients in the first few months of this pandemic, hospitals around the world are now turning their attention to signs of lasting damage, not just to the lungs but also the heart and other organs. CMR is emerging as a key tool for these investigations.
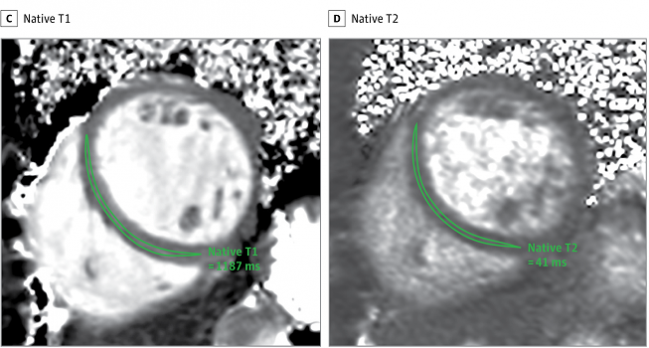
As Puntmann stressed to TCTMD, conventional CMR sequences were used for cardiac function, volumes, mass, and scar imaging, but a specific protocol was employed for myocardial T1 and T2 mapping. “Routine cardiac MRI can be a lot of things,” she said. “What we do and the way we go about our measurements is an obsessive-compulsive standardization exercise. We have worked very hard on making sure our measurements are robust, so that when we repeat them in the same patient, we actually can the determine the progress.” Ideally, for other groups to be able to perform the same type of follow-up in recovered patients they should adopt the same approach, she added.
Puntmann et al’s paper was published earlier this week in JAMA Cardiology.
Abnormalities on CMR
For the study, 100 patients who’d recovered from COVID-19 (median age 49) underwent high-sensitivity troponin T (hs-TnT) testing and cardiac MRI according to protocol. A minority of patients had traditional cardiovascular disease risk factors, and only one-third required hospitalization for their COVID-19 infection, with just two requiring mechanical ventilation.

At the time of CMR, 78% of patients had detectable hs-TnT despite delays ranging from 64 to 92 days since their positive COVID-19 test. Compared with risk factor-matched controls, the recovered COVID-19 patients had lower LV ejection fractions, higher LV volumes, higher LV mass, and raised native T1 and T2. Abnormal CMR findings in 78 out of the 100 COVID-19 patients included raised myocardial native T1 (73%), raised myocardial native T2 (60%), myocardial late gadolinium enhancement (32%), and pericardial enhancement (22%). There was a small but significant difference between patients who recovered at home versus in the hospital for T1 mapping, but not for T2 or hs-TnT. Time from diagnosis to CMR did not appear to influence the results.
“These findings indicate the need for ongoing investigation of the long-term cardiovascular consequences of COVID-19,” the authors conclude.
Within days of the JAMA Cardiology publication, however, Darrel Francis, MD, and Graham Cole, MBBS, PhD (Imperial College Healthcare NHS Trust, London, England), took to Twitter questioning some of the numbers in Puntmann et al’s paper. Specifically, Francis questioned whether key values were misreported as medians instead of means. Cole additionally raised the question of whether the patient whose histological and imaging findings are pictured in Figure 1 “was actually in the study” since a scatter plot in Figure 3c does not appear to include a data point for a patient with the same hs-TnT results at 78 days after diagnosis.
Contacted again by TCTMD, Puntmann said she is looking into the issues raised by Francis and Cole.
Cole, speaking with TCTMD, said that he’s had calls and emails from primary care physicians since reading the JAMA Cardiology paper, or the media coverage it generated, worried that they are not adequately investigating their COVID-19 patients. “So the reach of this is huge,” he said. “Make no mistake, this is a very exciting paper and I'm a proponent of cardiac MRI—it's about 75% of my working life. The problem as I see it is, whilst this is tremendously exciting research by a respected group of researchers, a lot happens on trust. When things that appear to be relatively simple cannot work, how do you trust the things that you can't check? That's the challenge here. It's trust but verify, and when you verify and it doesn’t work, you should ask for more detailed information.”
TCTMD reached out to James Carr, MD (Northwestern University, Chicago, IL), president of the Society for Cardiovascular Magnetic Resonance, who said he was aware of the questions being raised about the numbers reported in the JAMA Cardiology paper. To him, though, the cardiac abnormalities picked up using standard CMR as well as the T1 and T2 mapping appear undeniable, Carr commented.
“I think what's striking is the high percentage of abnormalities in these patients, many of whom were recovering from COVID at home and for quite a lengthy period afterwards, and I don't think this is really reflected entirely in this discussion about the values and whether they are medians or means,” he said.
“If you look at the delayed enhancement of myocardium, at least a third of the patients had abnormal myocardial enhancement,” quite apart from the more general figure of 78% for any abnormality on CMR, he pointed out. “There is clearly an impact going on in the heart, and I think that's probably indisputable.”
The dispute about means, medians, and interquartile ranges, Carr continued, “I’ll leave to the statistical experts to debate.” The T1, T2 results, often referred to as “imaging biomarkers,” are high and abnormal, he agreed. Moreover, the protocol used by the authors is well established, with “a lot of literature supporting its use,” particularly in the setting of infiltrative cardiomyopathies, but also in the evaluation of heart failure and ischemia. And in the paper, the addition of T1 and T2 mapping added incrementally to findings gleaned from a basic CMR protocol.
“So yes, I would agree that if you are going to run a protocol for evaluating patients for any kind of inflammation, it should be a combination of the basic protocol, which is cine imaging and late gadolinium enhancement, plus T1 mapping and T2 mapping,” Carr said.
What to Do?
Speaking with TCTMD before the questions were raised about the study numbers, Puntmann acknowledged that it’s still not clear what should be done when abnormalities are detected or how long they’ll last. She and her colleagues have research proposals in the works looking at the use of both heart failure medications and anti-inflammatories for cardioprotection. While it’s too soon to say, she stressed, she fears that while anti-inflammatories may be needed only in the short term, heart failure medications may be needed over the longer term.
These are the questions now rising to top of mind at hospitals now emerging from the first wave of the pandemic.
“We were very busy so far in terms of COVID-19 with the acute presentation: we haven't actually got our heads around the work that comes afterwards, and this is the next step. That's why I think our results are so important,” she said, because they offer a hint that some degree of inflammation is ongoing.
Since sending this paper for publication, Puntmann and her co-investigators have expanded the number of CMR tests in their series to 150 and those with the most intensive inflammation on the first test have already come back for follow-up CMR. “Most of them have still got ongoing inflammation that is of equal intensity,” she said, adding that they have not yet published this finding. “I would like to have a few more to really convince myself that this is what we're looking at,” said Puntmann.
Whether that inflammation then also translates into more-permanent damage is unknown, she noted. “We don't have the outcome data yet, but I always draw parallels with, for instance, swine flu, because that was also one of those moments where all of a sudden we saw an amazing amount of myocarditis and then obviously heart failure. For me, this is a little bit of déjà vu. I expect that COVID is also going to be an important driver of inflammatory cardiomyopathies and heart failure in the future, and given that this is happening at such a huge scale, we really need to act fast. We need to start making sure that we are diagnosing people early and intervening early.”
For this to work, she added, centers may need to move away from a reliance on cardiac biopsies, which are difficult to do early and on a large scale, particularly in the time of COVID-19. Instead, CMR should be employed more widely to help diagnose myocarditis early on. Moreover, Puntmann said, “we need to try to find a means that everybody can use. And then for my colleagues, for cardiac MRI experts, we need to agree on using the methods that can deliver this.”
Carr made the point that CMR is not widely used and is not currently recommended in COVID-19 patients, especially in the acute setting, but that he believes it could play a wider role and that Puntmann et al’s study will be the first of many trying to use this tool.
As to what can be done about the abnormal results, Carr stressed that he’s not a cardiologist. But he said that the chemotoxicity experience carries some useful lessons that could potentially be applied to the COVID-19 patients—a point also made by Puntmann. Myocarditis secondary to cardiotoxic medications used in cancer is known to predict later heart failure; research has shown that the use of cardioprotective medications may help mediate this damage.
“In COVID-19, you could potentially argue particularly in younger patients that if they have abnormalities on imaging, that maybe they could benefit from having cardioprotective medication started,” Carr said. “But again, there's no evidence to support that right now. I think one of the things that this paper will do is set the tone, set the stage, for doing some of these subsequent studies in patients who’ve had prior infection with COVID-19 and then seeing which ones go on to develop cardiac problems and whether medications can help to prevent that.”
Cole, asked whether he felt Twitter was the best place to air his concerns, argued that contacting authors and journals about errors and questions can sometimes be an “unfulfilling task.” In this case, he said, the questions have generated a lot of online discussion that will hopefully have an impact beyond this particular paper, given the minority of physicians trained to look closely at statistics. The best solution, he said, would be for the authors to make the anonymized data set available for others to review, or otherwise address the questions swiftly and transparently. “I really hope the authors in this case will help clarify. I would argue that within 24 hours we have got a fair amount of engagement and people thinking about it—it’s the kind of hive mind concept that can be beneficial. Twitter is a forum that doesn't have the shackles of traditional medical publishing, which doesn't really work for this kind of discussion.”
Puntmann, in an email to TCTMD after this story was published, observed: “Scrutiny is good and we are looking at the comments raised, [but] we fully agree with your questioning about Twitter being the right place to scrutinize this.” A letter to the editor, she added, “would indeed be more appropriate.”
Photo Credit: Puntmann, et al. Modified from: JAMA Cardiology. Used with permission of the authors and journal.
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Puntmann VO, Carerj L, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;Epub ahead of print.
Disclosures
- Puntmann reports support by grants from the German Ministry of Education and Research via the German Centre for Cardiovascular Research (DZHK) partner site RheinMain, Deutsche Herzstiftung e.V., Bayer, and Cardio-Pulmonary Institute.


Comments