In Transporting Donor Hearts, SherpaPak Shows Promise Over Traditional Coolers
Compared with cold storage, the organ-preservation system helped posttransplant outcomes despite longer ischemic times.
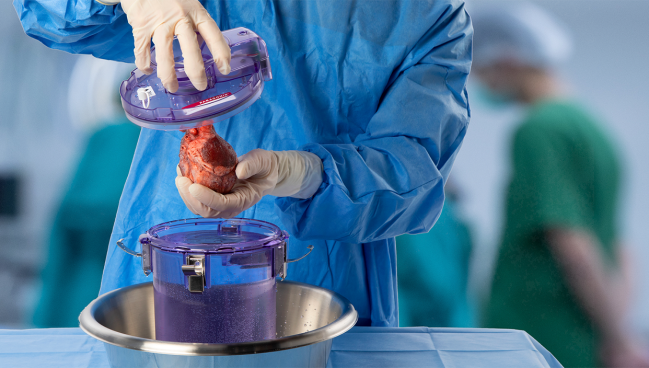
Photo Credit: Paragonix Technologies
Patients with a durable left ventricular assist device (LVAD) who receive a heart transplant have less primary graft dysfunction (PGD) when a dedicated system, rather than traditional cold storage, was used to transplant their donor organ under temperature-controlled conditions, new registry data suggest.
Traditionally, donor organs are locked in plastic bags with preservation fluid after harvesting then transported from the donor to the recipient hospital on ice in a simple cooler similar to what’s used for picnics. The SherpaPak (Paragonix) is one of a few new hypothermic systems that offers temperature control designed to minimize ischemic and cold-induced injuries to donated organs in transit.
“This study adds to the growing body of evidence that SherpaPak as well as other novel donor-heart-preservation systems can help to reduce primary graft dysfunction and severe primary graft dysfunction and that those methods of donor heart presentation are very likely to improve post heart transplant outcomes,” lead author Joseph B. Lerman, MD (Duke University Hospital, Durham, NC), told TCTMD. “This is particularly exciting because patients with durable LVADs are known to be some of the highest-risk recipients for heart transplant in terms of early post-heart transplant complications.”
The hypothesis behind the study, he explained, is that LVAD patients who are at higher risk for PGD, a primary cause of early morbidity and mortality posttransplant, might see the most benefit from a device like SherpaPak since the hearts would arrive in better shape than they might using traditional cold storage.
This kind of tool also holds great potential for increasing the donor pool generally, he added. “It's such a shame that every year there are donor hearts which go wasted while we have patients waiting for them. So technology that can let us go farther to utilize these hearts safely I think is so important.”
The increasing use of durable LVADs has improved survival for patients who need them over the last several years, but this practice “has created a bit of a dwindling access to organs for folks who are awaiting transplant,” Sounok Sen, MD (Yale School of Medicine, New Haven, CT), who was not involved in the study, told TCTMD. “What this study, to me, demonstrates is that advancements in organ preservation technologies help mitigate some of the effects of challenges that our patients on durable LVADs face while awaiting transplant.”
In particular, he added, the ability of organs to travel from greater distances and maintain quality over longer ischemic times without resulting in more PGD would be "very important advancements for our field.”
Less PGD With SherpaPak
For the study, which was published last week in Circulation: Heart Failure, Lerman and colleagues included 327 patients with durable LVADs from the Global Utilization and Registry Database for Improved Heart Preservation-Heart (GUARDIAN) Registry who received a heart transplant using traditional cold storage (n = 178) or the SherpaPak (n = 149) at one of 14 US centers between October 2015 and August 2022.
With the SherpaPak, the donor heart is attached to a sterile cannister connected to the aorta and then the cannister is filled with cold preservation solution, sealed, and placed into an outer container that contains phase-change material to create a uniform cooling system. The organ temperature is continuously logged using a wireless monitor with the device aiming to preserve temperatures in the range of 4-8° Celsius.
Patients in the SherpaPak group were more likely than those whose hearts were transported using traditional cold storage to undergo heart transplant after 2018 when the organ allocation policy changed (96.6% vs 60.1%; P < 0.001), but also had worse baseline IMPACT scores (mean 6.7 vs 5.3; P = 0.007) and more use of extracorporeal membrane oxygenation (ECMO) or temporary LVAD pretransplant (10.1% vs 3.4%; P = 0.022). Lastly, distance to organ (mean 404 vs 242 miles) and total cold ischemic time (mean 215.8 vs 192.7 minutes) were longer in the SherpaPak arm (P < 0.001 for both).
After transplant, use of ECMO or temporary LVAD was less common for SherpaPak patients compared with cold storage (6% vs 15.2%; P = 0.012), and the groups reported similar usage of intra-aortic balloon pump.
In analyses adjusted for age, IMPACT score, total ischemic time, and temporary mechanical circulatory support use, SherpaPak use was associated with less PGD than traditional cold storage (OR 0.56; 95% CI 0.32-0.99) as well as severe PGD (OR 0.31; 95% CI 0.13-0.75). Severe PGD is defined as the need for left or biventricular mechanical support including ECMO, LVAD, or biventricular assist device use in the first 24 hours after transplant.
In a propensity-matched analysis, the researchers reported trends toward a shorter ICU (7.5 vs 11.3 days; P = 0.09) and total hospital stay (20.5 vs 28.7; P = 0.06) with SherpaPak compared with cold storage.
Notably, both 30-day survival (96.6% vs 96.6%; P < 0.99) and 1-year survival (91.9% vs 90.3%; P = 0.69) were similar between the SherpaPak and traditional cold storage groups.
Mechanisms at Play
Transplant teams have long been using ice and coolers to “make the heart metabolically inert, so that those cells of the heart that are no longer receiving oxygen and nutrients from the bloodstream of the donor can experience as minimal damage as possible from the time that it's removed from the donor and transplanted into the recipient,” Lerman explained. “The problem with putting the hearts in a standardized cooler, though, is that you have very little ability to control the temperature that the heart might actually reach.”
Because an organ can suffer irreversible damage when kept at less than 2° Celsius, he said the promise of a device like SherpaPak would be that it improves heart function after implantation given the consistency with which it keeps the temperature during transport. That said, Lerman admitted he was surprised not to see a survival benefit in this study and said the prospective registry design is susceptible to residual confounding that might have factored in.
Transplant teams that can travel further for organs are going to be able to perform more transplants and help more patients on the wait list. Joseph B. Lerman
Interestingly, Sen noted that the incidence of both 30-day and 1-year mortality was “a lot more than other registries and other studies have reported, . . . around 30-45% lower for LVAD patients in particular.” He added that the prospective-registry design might have enrolled patients that “may not necessarily reflect the national sample.”
Still, despite the “significant increase in distance to organ in the SherpaPak arm, [the fact that] they still had a reduction in PGD is a very important finding,” Lerman said. “Transplant teams that can travel further for organs are going to be able to perform more transplants and help more patients on the wait list. And [for] transplant surgeons who can spend more time in the operation ensuring that they can achieve hemostasis in difficult patients like adults with congenital heart disease or patients with durable LVADs . . . without having to worry as much of the effect of ischemic time on primary graft dysfunction, that's a tremendous benefit to your surgeons as well.”
But the mechanism with which these devices work hasn’t totally been clarified. A similar system manufactured by XVIVO, which was recently reported in the Lancet to have preserved a donor heart for more than 12 hours aboard a commercial flight from the French West Indies to Paris, also maintains a precise donor heart temperature range, but additionally provides oxygenated blood mixed with electrolytes and other agents throughout transport.
“To really isolate the benefits of that temperature range versus some of those other things that are done in the XVIVO,” a randomized trial comparing it with SherpaPak might be useful, Lerman said. “But my assumption is that the benefits you're seeing really do come from that more-precise temperature control and minimizing the not only ischemic time but also the cold-induced graft injury that happens in a traditional cold storage model.”
Worth the Greater Cost?
Cost is the big elephant in the room for these devices, however. Lerman estimated that a single SherpaPak use can cost between $9,000 and $17,000, which is substantially greater than filling up “a cooler that you may have in your garage” with ice.
“Though that $17,000 certainly is expensive, . . . a device that gets a patient out of the ICU 2 days quicker, that prevents them from needing ECMO or hemodialysis, certainly is going to pay for itself in terms of the savings generated to the health system,” he said, adding that randomized trials are needed to confirm these benefits.
Sen agreed that questions around cost in this setting are “complicated.” He said: “We have to keep costs in context with what our goals are for our patients and understand if that makes sense from a societal level.”
At his institution, where they currently use both the SherpaPak and XVIVO device, “the components that we put into our calculation [for how to transport organs] include recipient level complexity . . . and then also distance and what our anticipated ischemic time is,” Sen said. “So the findings from this study help corroborate some of the things that we think of every day.”
He agreed with Lerman on the necessity of a randomized trial comparing the available devices to “help us as clinicians ensure that we're making the right decision when we're adopting these new technologies.”
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Lerman JB, Patel CB, Casalinova S, et al. Early outcomes in patients with LVAD undergoing heart transplant via use of the SherpaPak cardiac transport system. Circ Heart Fail. 2024;17:e010904.
Disclosures
- Lerman reports receiving supported from the National Institutes of Health.
- Paragonix Technologies funds and administers the Global Utilization and Registry Database for Improved Heart Preservation-Heart Registry and provided statistical support for this study.
- Sen reports no relevant conflicts of interest.




Comments