AI Assessment of Plaque, Hemodynamics May ID Lesion-Specific ACS Risk
This brings the field closer to selecting the best patients to undergo PCI to stave off future events, Andrew Choi says.
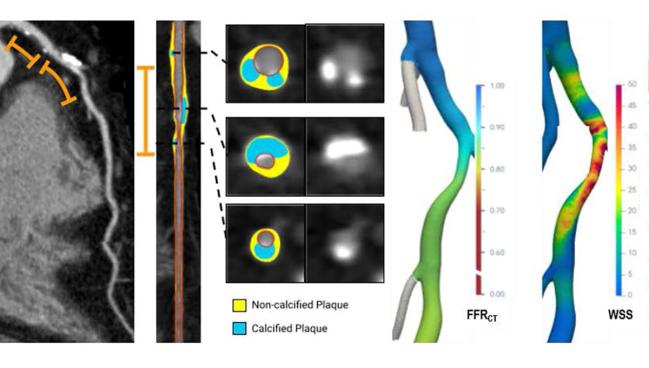
Photo Credit: Adapted from Koo BK. Artificial intelligence-enabled quantitative plaque and hemodynamic analysis for predicting acute coronary syndrome risk and prevention strategy: the EMERALD II study. Presented at: TCT 2023. San Francisco, CA.
WASHINGTON, DC—An artificial intelligence-enabled quantitative coronary plaque and hemodynamic assessment (AI-QCPHA) applied to coronary CT angiography (CCTA) may help identify which patients with ischemic lesions are likely to benefit the most from undergoing PCI to stave off a future ACS event, an analysis of the EMERALD II trial suggests.
Applying the technology to vessels with a low CT-derived fractional flow reserve (FFRCT), Seokhun Yang, MD (Seoul National University Hospital, South Korea), and colleagues identified five high-risk features involving luminal stenosis, atherosclerotic burden, local hemodynamic severity, and myocardial blood flow that were associated with subsequent culprit lesions.
“In ischemia-causing lesions considered for PCI, lumen, plaque, and hemodynamic features collectively contributed to the risk of future ACS,” Yang told attendees of the recent 2024 Society of Cardiovascular Computed Tomography (SCCT) meeting. “Integrative assessment of them using AI-QCPHA can optimize selective PCI planning at the up-front stage.”
This analysis is addressing “the Holy Grail of cardiology,” commented Andrew Choi, MD (George Washington University School of Medicine, Washington, DC), who was not involved in the study. “How can we identify the vulnerable plaque and treat it before the heart attack happens? This is something we've been [exploring] for the last 30 years.”
Before there were AI and machine-learning algorithms, analysis of plaque and hemodynamic features was done on a semiautomated or manual basis with simple binary assessments (eg, whether there is high-risk plaque present or the FFRCT is showing ischemia).
Now, with AI-QCPHA, “they are combining not only the presence or absence of significant FFRCT, but also the change in FFRCT. They're also looking at conventional features on CT, like diameter stenosis, which is important. And then they're also adding a quantitative plaque assessment, which I think is key, and with a particular focus on total plaque burden and noncalcified low-density plaque,” Choi said. And then they’re integrating all of that information together in “a meaningful way to be able to help predict ACS.”
Finding High-risk Features
Clinical guidelines recommend revascularization of ischemic vessels (FFR ≤ 0.80) to improve outcomes in patients with stable coronary lesions. A meta-analysis of three randomized trials, however, showed that most patients treated medically—though they have worse outcomes overall compared with those treated with FFR-guided revascularization—will not have a hard outcome event.
“This suggests the necessity of a selective patient strategy rather than PCI on all patients with low FFR,” Yang said.
Moreover, a recent study demonstrated that among patients with ischemic vessels (FFRCT < 0.80), revascularization was associated with fewer cardiac events compared with medical therapy only in the presence of high-risk plaque features, highlighting the need to identify specific predictors of future ACS events in ischemic vessels.
To address this question, Yang et al examined data from the EMERALD II trial, in which AI-QCPHA had prognostic value for ACS in patients who underwent CCTA and subsequently developed ACS 1 month to 3 years after the scan. All patients underwent invasive angiography at the time of ACS to define the culprit lesion, and these results were compared with the initial CCTA that included the AI-QCPHA analysis.
The current analysis included 287 patients (mean age 67 years; 73% male) who had 1,527 lesions in ischemic vessels, including 264 culprit lesions and 1,263 nonculprit lesions. The median time between the initial CCTA and the ACS event was about 1 year; 25% of patients had STEMI, 37% NSTEMI, and 38% unstable angina.
The culprit lesions were more likely to have at least two adverse plaque characteristics (46.2% vs 18.4%) and to have severe stenosis (41.7% vs 10.7%; P < 0.001 for both).
Applying AI-QCPHA revealed many plaque and hemodynamic features that differed between culprit and nonculprit lesions, with the researchers identifying five as being the most important when predicting future ACS risk (with cutoffs selected to maximize sensitivity and specificity):
- Percent total myocardial blood flow ≥ 20% (56% of patients)
- Change in FFRCT ≥ 0.05 (42% of patients)
- Percent area stenosis ≥ 65% (40% of patients)
- Noncalcified plaque volume ≥ 72.5 mm3 (39% of patients)
- Plaque burden ≥ 85% (35% of patients)
The combination of those features provided an area under the curve (AUC) of 0.837 for identifying risk of an ACS event, which was higher than the performance of the degree of stenosis plus the number of adverse plaque characteristics on the standard CCTA evaluation (AUC 0.781; P < 0.001).
Patients with at least three of those AI-QCPHA features were considered to be in the high-risk subset, which encompassed 38% of the cohort.
Searching for the Holy Grail
How to identify which lesions to treat is a question that has gained even more interest following the release of the PREVENT trial results earlier this year, Choi said. The results showed that patients randomized to prophylactic PCI to treat vulnerable plaques identified on invasive imaging versus optimal medical therapy alone had a lower risk of serious CV events over the next 2 years.
“That's an important trial. Can we do this with noninvasive imaging?” Choi said, adding, however, that “the PREVENT trial data have to be validated and replicated in a different cohort before there's acceptance.”
As for the current analysis of EMERALD II, there are some limitations, Choi said, pointing to the remaining need to take the study to the next step and evaluate the impact of treatments. In addition, the comprehensive AI-QCPHA analysis used here—which relies on a proprietary algorithm—is dependent on excellent image quality and is still in an early validation stage.
Even so, Choi said, the study “does show us that it's integrative analysis—it's the combination of stenosis, quantitative plaque burden, and flow—that moves the needle and gets us closer to identifying the right . . . patients that we can treat.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Yang S. Coronary CT angiography-derived precursors of acute coronary syndrome in ischemia-causing lesions. Presented at: SCCT 2024. July 20, 2024. Washington, DC.
Disclosures
- Yang reports no relevant conflicts of interest.
- Choi reports consulting fees/honoraria from Siemens and stock options from Cleerly.





Comments