Amulet IDE: LAA Occluder Performs Well Against Watchman
The Amplatzer Amulet device provided better closure as well as noninferior safety and effectiveness.
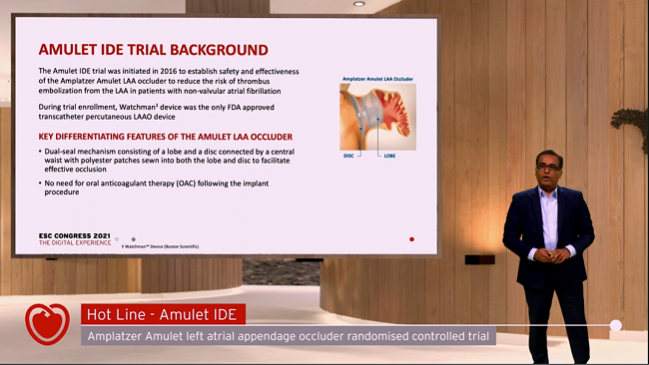
(UPDATED) In patients undergoing left atrial appendage (LAA) occlusion, the Amplatzer Amulet device (Abbott) provides better closure as well as noninferior safety and effectiveness compared with the first-generation Watchman device (Boston Scientific), results of the Amulet investigational device exemption (IDE) trial show.
The device met all three primary endpoints assessing the completeness of the seal at 45 days, safety at 12 months, and effectiveness in terms of rates of ischemic stroke or systemic embolism at 18 months, Dhanunjaya Lakkireddy, MD (Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, KS), reported during the virtual European Society of Cardiology Congress 2021.
There were more procedure-related complications in the Amulet arm of the trial (4.5% vs 2.5%), driven by increases in pericardial effusion and device embolization, but Lakkireddy said rates declined as operators gained experience with the new device.
Of note, most patients treated with the Amulet were discharged on antiplatelet therapy alone, with only 21.1% receiving oral anticoagulation. In contrast, nearly all patients treated with the Watchman (95.8%) were discharged on an anticoagulant per the instructions for use.
“The Amulet left atrial appendage occluder can safely and effectively treat more nonvalvular atrial fibrillation patients with the option to discharge without needing oral anticoagulation,” Lakkireddy said at a media briefing. “Its immediate closure to reduce the risk of stroke and immediate freedom from oral anticoagulation could be attractive.”
Indeed, Gerhard Hindricks, MD, PhD (Leipzig Heart Center, Germany), one of the chairs of the European AF guidelines, said of the ability to discharge patients on antiplatelet therapy alone that “procedure-wise and also from a patient perspective, it’s a significant step forward.”
Overall, he said after Lakkireddy’s presentation, the Amulet IDE trial was well planned, designed, and executed. It’s important “because it brings solid data to a field where data are really lacking,” he added, noting that it is the largest trial of LAA occlusion performed to date.
Still, the trial doesn’t provide information on the relative safety and efficacy of LAA occlusion versus direct oral anticoagulants (DOACs), and a direct comparison is “urgently needed,” said Hindricks, who pointed out the rates of stroke, TIA, and device-related thrombus (DRT) were higher than expected in this trial.
The findings led to US Food and Drug Administration approval of the Amulet earlier this month, increasing options for LAA occlusion among US physicians who had been restricted to use of the first-generation Watchman and new-generation Watchman FLX.
Amulet IDE Trial
The Amulet consists of two components to close off the LAA—a lobe that plugs the neck of the appendage connected to a disc that seals off the orifice. The Watchman devices, in contrast, have a single component to occlude the appendage.
The Amulet IDE trial, started in 2016, was designed to compare the two device types. Investigators randomized 1,878 patients (mean age 75; about 60% women) who had atrial fibrillation; had a high risk for stroke or systemic embolism; were suitable for short-term warfarin therapy but deemed unable to take chronic anticoagulation; and had imaging results that indicated either device could be implanted. The average CHA2DS2-VASc score was 4.5 to 4.7, the average HAS-BLED score was 3.2 to 3.3, and most patients (72%) had a history of major or minor bleeding. Roughly another 30% had a prior history of stroke/TIA. All patients in the Watchman arm received the first-generation device, as the Watchman FLX was not commercially available by the end of trial enrollment.
Discharge medications included aspirin plus clopidogrel or aspirin plus an oral anticoagulant in the Amulet arm and aspirin plus warfarin in the Watchman arm.
The success of the initial implantation procedure was slightly higher in the Amulet group (98.4% vs 96.4%). At 45 days, the proportion of patients who had a residual jet around the device of 5 mm or less as seen on a transesophageal (TEE) echocardiogram also favored the Amulet (98.9% vs 96.8%; P < 0.0001 for noninferiority and 0.0025 for superiority).That difference, though statistically significant, has uncertain clinical relevance, Hindricks commented.
The primary safety endpoint was the composite of procedure-related complications, all-cause death, or major bleeding at 12 months, and the rate was comparable in the two arms (14.5% with the Amulet vs 14.7% with the Watchman; P = 0.0002 for noninferiority).
The primary effectiveness endpoint—a composite of ischemic stroke or systemic embolism—was assessed at 18 months; the rate was 2.8% in each arm (P < 0.0001 for noninferiority).
Despite less use of oral anticoagulation in the Amulet-treated patients, the rate of device-related thrombus (DRT) at 18 months was lower in that group (3.3% vs 4.5%). Two patients who had a DRT had an ischemic stroke, both in the Watchman arm.
Also at 18 months, the rate of all-cause death was lower in the Amulet group (6.7% vs 9.6%), with similar TIA and hemorrhagic stroke rates with each device.
Major bleeding was seen in 11.6% of Amulet-treated patients and 12.3% of those who received the Watchman, a nonsignificant difference. But that level of bleeding overall seems high, Renato Lopes, MD, PhD (Duke Clinical Research Institute, Durham, NC), commented during a panel discussion. “When we choose a treatment for these patients, we really want to minimize bleeding and preserve the efficacy so we can find the sweet spot,” he said. “But if the bleeding risk here seems to be high, how can we incorporate it in practice?”
Lakkireddy explained the bleeding rates by noting that the trial required participants to be eligible for short-term anticoagulation but poor candidates for longer-term therapy, and also that most of the patients had a prior history of bleeding and were therefore prone to those types of complications. He said bleeding rates would have been much higher if all of these patients had continued taking anticoagulation rather than undergoing LAA occlusion.
Importance of Having Options
Commenting for TCTMD, Luigi Di Biase, MD, PhD (Albert Einstein College of Medicine at Montefiore Hospital, Bronx, NY), a member of the American College of Cardiology’s Electrophysiology Council, touted the benefits of having multiple options when it comes to LAA occluders, noting that the appendage comes in many shapes and orientations. Different devices may be better suited to certain anatomies, and “having the option of being able to choose between one device over the other, it’s a great thing,” he said.
Digging into the findings, Di Biase said it was “very satisfying” to see that the Amulet came with a minimal number of leaks around the device at 45 days. Although there is debate among electrophysiologists about what size leak is important, “I personally [think] that any leak is an issue because you have to prevent stroke by occluding the appendage and if it is not well occluded, it’s an issue.”
Any leak is an issue because you have to prevent stroke by occluding the appendage and if it is not well occluded, it’s an issue. Luigi Di Biase
As for the comparison between the two trial arms showing better closure with the Amulet, Di Biase noted that it was the first-generation Watchman used in the trial and not the Watchman FLX, which is associated with fewer leaks. “I’m not convinced that when compared to FLX, there will be less leaks at implant” with the Amulet, he said. Nonetheless, “this device has proven to have a [minimal] number of leaks at implant and therefore it’s a very good device.”
Another positive finding, he said, is that despite differences in antithrombotic regimens at discharge, there were no major differences in safety or clinical outcomes. “The fact that even from the implant date you don’t have to be on an oral anticoagulant, but you can be on dual antiplatelet therapy, without this increasing the risk of TIA, stroke, and device-related thrombus is very, very interesting and satisfying,” Di Biase said, adding that it will be important to wait for the planned 5-year follow-up to get a fuller picture.
He said something to consider when interpreting these data, however, is that the older Watchman device was used in conjunction with warfarin at discharge in this trial, so it remains unclear how the Amulet plus antiplatelet therapy at discharge would perform against the more-contemporary approach of implanting the Watchman FLX and using a DOAC at discharge.
At this point, Di Biase said he’d feel comfortable using either type of device, but particularly in patients who have a very high bleeding risk and who can’t be on even short-term anticoagulation, the Amulet is a good option “because it has been proven in a randomized trial to be absolutely safe.”
He underscored the importance of having options, as it will improve the care provided to patients. “Of course every [device] selection needs to be made based on patient profile, patient characteristics, shape of the appendage, orientation of the appendage, number of lobes,” he said. “All this information will be key for physicians to determine which device they want to implant.”
Lakkireddy said it’s recommended to have a CT or TEE image to help in device selection before the procedure. “Now with Amulet being approved, preprocedural imaging becomes even more relevant so that you can make the choice ahead of time going into the case,” he advised.
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Lakkireddy D. Primary outcomes of the Amplatzer Amulet IDE randomized controlled trial. Presented at: ESC 2021. August 30, 2021.
Disclosures
- The Amulet IDE trial was funded by Abbott.
- Lakkireddy reports consulting for Abbott, Biosense Webster, Boston Scientific, Philips, Stereotaxis, and AtriCure.
- Di Biase reports being a consultant for Stereotaxis, Biosense Webster, Boston Scientific, Abbott Medical, and Rhythm Management; and having received speaker honoraria/travel support from Biotronik, Medtronic, AtriCure, Bristol-Myers Squibb, and Pfizer.



Comments