Bruises and Bumps: How Cardiology Meetings Survived the End of Direct Industry Sponsorship in Europe
In the wake of new regulations, a feared plunge in meeting attendance wasn’t seen and, in some cases, registration rose. But what’s next?

Organizers of major cardiology conferences in Europe and the United States expected a whole lot of discomfort when MedTech Europe, a trade group representing the medical technology companies, phased out direct industry sponsorship of healthcare professionals to attend conferences and other continuing medical education (CME) activities at the beginning of 2018.
But looking back at this first year under the new rule, the big general and interventional cardiology meetings seemed to do just fine, for the most part maintaining or even increasing attendance numbers. Attendance at some meetings went down, but not by half as some had predicted heading into the year.
“It has not been as bad as everybody feared it would be,” said Isabel Bardinet, CEO of the European Society of Cardiology (ESC), which holds a main congress and several smaller meetings each year.
It has not been as bad as everybody feared it would be. Isabel Bardinet
Bardinet told TCTMD there are several reasons why attendance at meetings didn’t drop as precipitously as expected. The fact that not all device companies have applied the code consistently could have played a role, she said, but it could also be that the international reach of meetings—the ESC Congress attracts close to 30% of delegates from the Asia-Pacific region, for example—dampened the effects of the new European regulations.
“We’re lucky in the sense that we’re very international,” Bardinet said. “We’re not totally reliant on the device industry except for one congress”—the European Heart Rhythm Association (EHRA) Congress—“so I think overall it’s been spread out in such a way that it hasn’t impacted as badly as we feared.”
Other conference organizers in Europe and the United States echoed the belief that attendance did not nosedive nearly as steeply as predicted and described proactive efforts to head off any potential adverse effects of the new policy. As the first year under the policy comes to a close, though, questions remain about how the system might be made smoother and about the long-term impacts of the rule, particularly regarding any effects on how physicians continue to be educated and trained—and how that might impact patient outcomes.
‘Arms’-Length’ Funding of CME
MedTech Europe adopted its new code of ethical business practice in December 2015. The code, which provides rules for how healthcare professionals and organizations can interact with industry, addresses many topics, but the main point of contention for many was the elimination of direct industry sponsorship for healthcare providers attending educational events organized by third parties. Under the new rules, direct support is still allowed for third-party-organized procedure-training meetings or company-sponsored satellite symposia if there is a consulting agreement. Industry continues to provide money for educational purposes, but it’s given to medical societies, professional congress organizers (PCOs), and hospitals for distribution.
In a pamphlet designed for healthcare providers, MedTech Europe explained its reasoning: “The new code is a clear message from the medical technology industry that we want to safeguard and protect our relationship with healthcare professionals by adopting a clear and strict self-regulation. Our industry is still fully committed to support independent medical education. We will now do this at arms’ length through independent third parties. The independent third party will decide [who will] receive the funding.”
Although that’s how industry funding of CME has been handled in the United States for many years, the rule represents a culture change in Europe. MedTech Europe delayed full implementation of that provision for 2 years—to the beginning of 2018—to allow for a transition.
Despite having time to prepare, some physicians involved in organizing cardiology conferences predicted dire consequences. Before official adoption of the code, a group of prominent interventional cardiologists said attendance at meetings could drop 30% to 50%, with the resulting deficit in education ultimately endangering patient outcomes. They called for a delay of 1 more year; MedTech Europe rejected it, citing the fact that discussions about abolishing direct sponsorship had been ongoing for almost a decade.
What Actually Happened?
It seems those fears were overblown. At least so far, meeting organizers in Europe and the United States have been able to adapt to the new landscape without major impacts on attendance.
Of all the meetings ESC organizes, Bardinet said, only the EHRA Congress is heavily dependent on support from the device industry; device manufacturers account for 70% to 80% of companies that participate in that meeting, compared with about 10% for the ESC Congress.
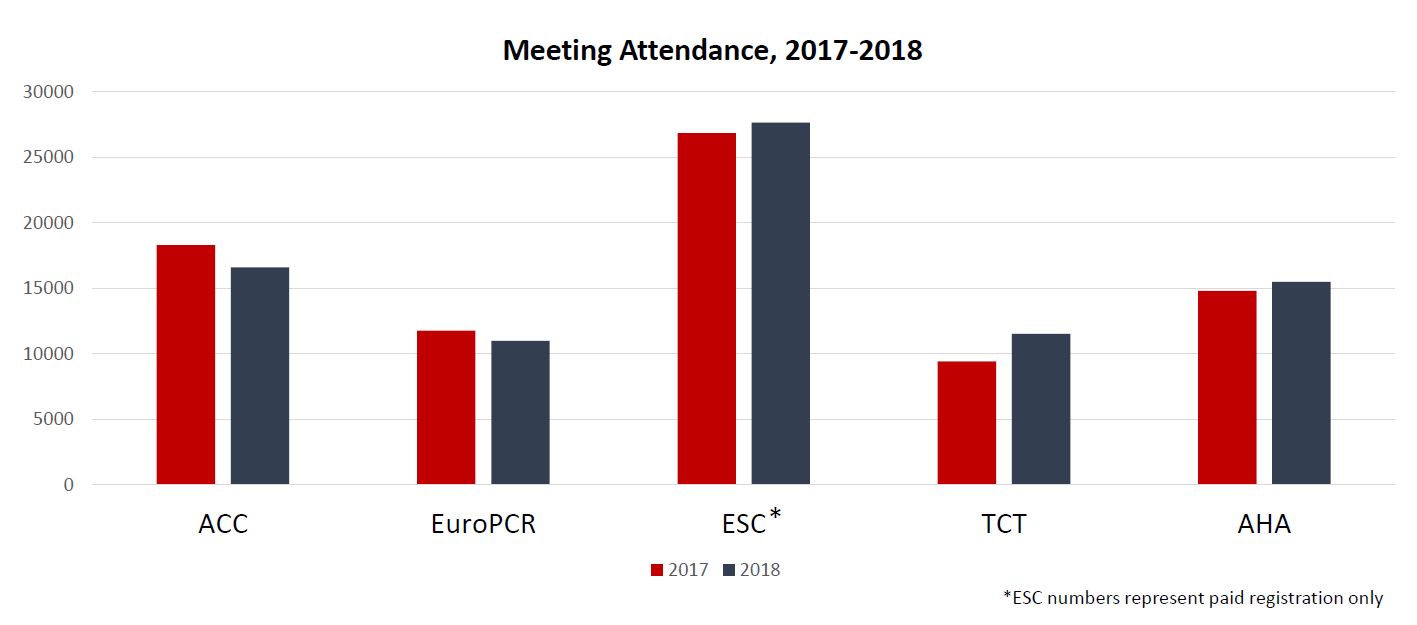
The main congress was unaffected by the new rule, and, in fact, had an increase in paid registrations over 2017 (from 26,857 to 27,663); most of the smaller meetings increased attendance as well, Bardinet said.
In contrast, EHRA—which already faced some challenges when it moved from June to March and shifted from a biennial to an annual meeting this year—did take a hit. The number of attendees sponsored by industry dropped by 30%, but overall attendance declined by only 15%. “So they have been finding other ways of coming to the congress. Not completely compensating for the drop, but partially,” Bardinet said. She predicted that attendance numbers would tick back up for EHRA 2019, pointing to initial signs from the number of abstracts submitted and rooms booked.
Bardinet noted that the minority of attendees coming to the ESC Congress or EHRA are dependent on industry funds. This year, only 7.2% of registrations for EHRA and 0.03% for ESC were paid for by industry grants. “We know, however, that industry supports many other physicians through grants to hospitals and local PCOs; this is not quantifiable at an industry level unfortunately as it is managed on a country-by-country basis,” she explained.
Results were mixed for EuroPCR and PCR London Valves, which are much more dependent on support from the device industry compared with the general cardiology congresses. Overall attendance declined by about 7% for EuroPCR compared with 2017, whereas it increased by about 3% for PCR London Valves.
Echoing Bardinet’s sentiment about the overall impact of the new rule, Patrick Jolly, director of PCR strategic & market development at Europa Group, a PCO based in Paris, France, said: “I think it has been not as bad as we thought it could have been.”
Jolly told TCTMD the new MedTech Europe rules could have contributed to the drop in attendance at EuroPCR, but noted that other factors might have been playing a role as well. For instance, the meeting fell during Ramadan this year and thus there was decline in attendees from predominantly Muslim countries.
Romain Despax, director of PCR activities for Europa Group, added, “It hasn’t been like the 30% drop that some people could have imagined before. It was really hard to tell at the beginning of the year how things were going to be and so it seems to be quite reasonably good turnout for these two major conferences in the field.”
US cardiology meetings also fared well in general. The largest interventional cardiology conference, TCT, had 11,538 attendees (up from 10,072 in 2016 and 9,429 in 2017). Total attendance for the American Heart Association (AHA) Scientific Sessions exceeded 15,500, up from about 14,800 in 2017. An AHA spokesperson said attendance from Europe was up from the previous year.
Bucking the attendance trend was the American College of Cardiology (ACC) Scientific Session, which saw overall attendance decline from 18,308 in 2017 to 16,609 in 2018. An ACC spokesperson said in an email, however, that “meeting attendance fluctuates year to year for many reasons, and we believe it’s premature to draw conclusions after only 1 year of data.”
[Even though it went] better than expected for the big conferences, for the small conferences, it’s a mess. Michael Haude
But Michael Haude, MD, PhD (Städtische Kliniken Neuss, Germany), who is immediate past president of the European Association of Percutaneous Cardiovascular Interventions and is involved with congresses under the PCR and ESC umbrellas, noted that even though it went “better than expected for the big conferences, for the small conferences, it’s a mess.”
Haude was referring to meetings in the 100- to 300-attendee range and lasting a day or day-and-a-half, which sometimes focus on issues of interest to physicians within a specific referral area. In contrast to the larger conferences, these types of meetings are not able to set up the needed infrastructure to handle the distribution of grants from industry, he said. And as the medical technology industry focuses its resources on bigger meetings, many of these smaller meetings will start to disappear, Haude said.
Implementation Challenges
Aline Lautenberg, director of legal affairs & compliance for MedTech Europe, described this implementation year as extremely busy for them. She said the code was initially drafted to be a set of principles, but the group soon realized that more practical guidance was needed both on the part of the device companies and medical societies, hospitals, and PCOs about how to put the rules into place. To that end, MedTech Europe has been organizing best-practice sessions and providing more guidance on the operational aspects of the code throughout the year.
“We thought it was already quite detailed, but actually moving to something new required much, much more guidance, much more exchange, much more dialog than what we expected in the first place,” Lautenberg told TCTMD.
Moving to something new required much, much more guidance, much more exchange, much more dialog than what we expected in the first place. Aline Lautenberg
On the side of meeting organizers, there was generally a need to dedicate resources and personnel to handle the grant process.
Bardinet said ESC had to set up a new system and dedicate two staff members to organize industry grants.
For PCR, Europa had to invest in building a team to handle the logistics of the new grant system, including selecting who would receive grant money, inviting them, and then dealing with all the administrative and regulatory issues involved with bringing them to the meeting. Despax noted that there is an administration fee added to pay for those services and that the team is continuing to look at ways to streamline the process and make it more sustainable.
“We had to be very proactive in order to make it happen as it happened. We did not rest on our laurels,” Jolly said. “If we had not done this then I think we would have suffered really quite a bit. We had to be very proactive in the months prior to EuroPCR to really see and be in control of this new process.”
As for whether these added efforts affected the bottom line financially, Jolly said: “We consider it an investment which is worthwhile, because this is also a way to provide access to the value of the course to as many people as possible. We had to change the model compared to what we used to do before.”
TCT, which came later in the year, also managed to avoid a downturn in attendance.

Juan Granada, MD, president and CEO of the Cardiovascular Research Foundation (CRF), which organizes TCT (and publishes TCTMD), said multiple factors contributed to lessening the anticipated blow from the new MedTech Europe rules. The rules forced physicians to make a decision about which meetings to attend, and they chose the bigger meetings, Granada said, adding that the location of TCT this year—San Diego, CA—was a draw. In addition, a change was made to the meeting, with a major investment put into providing more practical, hands-on training opportunities for physicians, which proved popular. And finally, CRF offered a reduced “special 30th anniversary” registration rate to entice attendees.
“When you look at the entire scheme of things, I really think it’s a combination of everything,” Granada told TCTMD.
Asked about sustainability moving forward, Granada pointed to the enduring educational needs in a field that is becoming more and more complex. “The need is there to train physicians in doing better procedures and using better devices. The need is absolutely there. The market size, the population is there,” he said. “[Whether] we’re going to be able to sustain this model from the financial point of view needs to be determined, but there is no question that the relevance of this meeting is there. From the clinical need point of view, it’s sustainable, but let’s see if the financial model actually can sustain itself.”
Overall Level of Investment
What also remains unclear is whether industry has maintained its overall level of investment in medical education.
Lautenberg told TCTMD last year that she didn’t expect the overall amount of money to change, although shifts might be seen in how funds are allocated.
One of the predictions made was that younger physicians, as well as nurses and technicians, would find it harder to attend meetings, and it appears that has come to fruition, at least for some meetings. Companies are still providing money for educational purposes, but they are often placing restrictions on the grants that put the focus on physicians and essentially exclude nurses and allied health professionals.
EuroPCR saw a decline in the number of nurses and allied professionals attending the meeting from 520 in 2017 to 456 in 2018, whereas PCR London Valves saw a slight increase—from 132 to 140. This year, however, the number of indirect grants made available for nurses to attend the meeting was only 12 for EuroPCR and five for PCR London Valves.
Recognizing the importance of getting these healthcare providers to meetings, Jolly said his team is working with the device companies to make more unrestricted grants available to them. In addition, EuroPCR is offering an early-bird rate to fellows, nurses, and allied professionals for the 2019 meeting to lower the grant packages for these attendees, and the Europa team is designing a plan to offer partial grant packages to offset some of the costs of traveling to the meeting.
From the clinical need point of view, it’s sustainable, but let’s see if the financial model actually can sustain itself. Juan Granada
As for whether the total amount of money spent by device companies on supporting medical education changed substantially, that’s not entirely clear, because meeting organizers don’t necessarily know how each attendee is paying to be there.
However, Bardinet said the level has likely fallen, based on the number of registrations paid for by grants from industry. “It’s not the size of their booths or the investment that they may make through advertising and satellite symposia,” she said. “This is based purely on the number of registrations that they are paying for.”
Haude said it is difficult to determine whether the total level of investment is the same because even though companies seem to be supporting the big meetings to a similar degree, they might be saving by not spending as much on the smaller meetings.
Asked about whether the overall level of support has wavered, Lautenberg said, “I think that it’s impossible for us to make any type of assessment. I think that investment in medical education can take many forms, and congress support is one of them. Our leadership has always been committed to continue to support medical education, and from the feedback we have received so far, it seems that our major partners didn’t see, even considering that it’s a test year, major issues. So I’d say that all in all it went pretty well, and we could probably assume that the level of investment has stayed more or less the same.”
As for whether the new rules effectively placed a firewall between industry and individual healthcare professionals, Lautenberg said she believes that has happened.
“From where we stand, it seemed the right thing to do and I think our leadership is still committed that that’s the right [thing] to do even if it has in certain countries some operational challenges,” she said. “We think that we finally managed to do that, yes.”
Granada, however, said the new MedTech Europe regulations have in fact reduced transparency by introducing complexity into the system. “Because right now, it’s very complicated, bureaucratic, it’s adding cost to the system and adding a lot of red tape at multiple levels,” he said. “I’m all for transparency, but actually somebody has to figure out a better way to do this.”
Implementation of the MedTech Europe rules is a first step, Granada added, “but I don’t really think that the system is as functional and as straightforward as people believe it is.”
Ensuring Opportunities for Continuing Education
Moving forward, meeting organizers can expect to see ongoing effects from the new MedTech Europe rules, Bardinet indicated.
“I think the change will continue. It will not be perhaps as brutal as was thought a year ago, but I think that the fact that [companies] are supporting financially doctors less to go to congresses is a trend which will continue,” she said, noting that the percentage of attendees at the ESC Congress who are paid for by industry has declined for the past several years.
“We actually started 5 or 6 years ago many campaigns to encourage doctors to come on their own funds or hospital funds or university funds to congresses, and we have developed a very strong policy around educational grants to attend congresses. As such I think that that culture is beginning to take,” Bardinet said.
“I think the real question is: where are the funds to pay for the further education of doctors? The hospitals do not have them in the budget. The health authorities and governments are turning a blind eye,” she continued. “That is the question, because they’ve always expected industry to pay and now industry is pulling out. So the real question is how are we going to fund the continuing education of doctors, which is fundamental for the patients.”
Healthcare professionals are going to have to pick up the slack themselves, Bardinet said, stressing that providing education to as many doctors as possible—so they can best treat their patients—remains paramount.
Maintaining a connection between industry and the clinical community also remains key.
“I think that we have to be careful that this does not stop innovation and collaboration with industry for the development of new products, which could be an unfortunate side effect,” Bardinet said.
Jolly said that a connection with industry remains important for continuing education as well. “The shift towards self-funding by physicians, I think it’s not yet here in that specific community, so we need to account for this and we need to have the support from industry to continue to do things as we do today,” he stressed.

Haude also advocated for maintaining ties with industry. “For me, the important thing is that we manage to get into a collaborative, productive discussion process with the med-tech industry, and not a clash,” he said. “A clash would mean a disaster.”
Meetings remain essential for continuing training and education, and organizing them would not be possible without industry funds in the absence of another source of money, Granada said.
If the meeting quality stays high, people will continue to attend, he said. “We strongly believe that if TCT continues to deliver high-quality content and hands-on training and great talks and a great meeting, people will continue to come and they will find a way to come.”
Kinks to Be Worked Out
In terms of how the whole system of managing interactions with industry is working, the meeting organizers interviewed by TCTMD say there remains room for improvement.
“It’s been very complicated because no two industry companies have applied the code in the same way,” Bardinet said.
She pointed out that the restrictions placed on grants vary, with some companies saying they’ll specify the town and country from which a supported physician should come—“which is basically coming down to giving the name”—while others are placing quotas per country.
After the EHRA Congress, Bardinet said, the ESC team met with some of the companies to request a more harmonized way of doing things, “because it’s not really being very helpful.”
Bardinet had advocated for more time to prepare before direct sponsorship was ended, and, after seeing how it went this first year, she said it would have been helpful to have that extra time.
Lautenberg acknowledged that there are still some operational issues to smooth over and processes to streamline, adding that MedTech Europe has been keeping lines of communication open both with its members and meeting organizers.
“We knew that this year wouldn’t be the easiest in the sense that everyone had to get used to a new system,” she said. “I think that it would be overambitious to think that we’re able to do it in 1 year. . . . We were hoping to make it a smooth transition, and hopefully I’d say in 2021 and 2022 we’ll be comfortable with the model and everyone will have adapted to this new system.”
It’s also an open question whether the expected downtick in registrations may still prove a reality in the coming years, particularly as meetings drop the incentives or special programming used in 2018 to help lure physicians.
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full Bio

Oscar Berg