DES Best for In-Stent Restenosis, but DCBs Also Viable
In the RIBS trials, Abbott’s old BVS had the highest risks of TLR, specifically beyond 1 year, and safety worries when used for ISR.
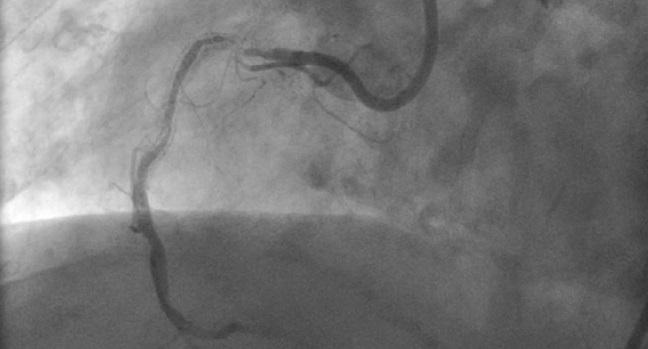
Photo Credit: Adapted from Kirtane AJ. ISR: Why the why matters. mechanism of action identification and ISR on IVUS. Presented at: Fellows 2024. Las Vegas, NV.
Two “leave-nothing-behind” strategies—either a drug-coated balloon (DCB) or the now discontinued bioresorbable vascular scaffold (BVS; Abbott Vascular)—result in similar clinical outcomes at 3 years when used for the treatment of in-stent restenosis (ISR), but both options fare significantly worse than a drug-eluting stent, an analysis of several trials from the RIBS series shows.
Beyond the 1-year mark, use of the bioresorbable scaffold was associated with a significantly higher rate of target lesion revascularization when compared with an everolimus-eluting stent (Xience; Abbott Vascular) as well as a greater risk of very late vessel thrombosis.
“In-stent restenosis still remains a major clinical problem,” senior investigator Fernando Alfonso, MD (Hospital Universitario de La Princesa, Madrid, Spain), told TCTMD. “Despite the advent of a new generation of drug-eluting stents, this will be the main cause of stent failure.”
According to US data, one out of 10 PCI procedures involve the treatment of at least one lesion with restenosis, said Alfonso. In Europe, the data suggest one out of every 20 PCIs involve the treatment of restenosis. “There is a little bit of discrepancy here, but [nonetheless] it is a major problem,” he said.
The latest European Society of Cardiology clinical practice guidelines recommend new-generation DES or DCBs for patients with ISR (class IA recommendation). Earlier this year, the US Food and Drug Administration approved the first coronary DCB (Agent; Boston Scientific) for the treatment of ISR based on the AGENT IDE trial. Abbott long ago stopped manufacturing their BVS following safety concerns, specifically the risk of scaffold thrombosis, but a disappearing scaffold has been suggested as a possible option for ISR because it wouldn’t leave more metal in the artery.
“New-generation BVS, with thinner struts, more radial force, and more uniform and reliable resorption will be a good strategy for patients with ISR to avoid a new permanent metal layer,” said Alfonso. “However, we learned our lesson, and these novel devices should be scrutinized. They should prove their safety and efficacy in well-designed, large RCTs, with long-term angiographic and clinical data.”
Three-Year Data From RIBS VI
The new analysis, published in the August 12, 2024, issue of JACC: Cardiovascular Interventions, includes longer-term follow-up of four studies from the RIBS investigators.
The analysis captures patients from RIBS VI and RIBS VI Scoring with ISR after receiving either BMS or DES who were subsequently treated with the first-generation BVS. In the randomized RIBS V study, patients were treated with either a DCB (Sequent Please; B. Braun) or everolimus-eluting stent following ISR after BMS implantation, while the RIBS IV study had the same design except but in patients with ISR after DES implantation. The 1-year results showed there were similar angiographic and clinical outcomes with BVS and DCBs, both of which were inferior to the everolimus-eluting stent.
At 3 years, clinical follow-up captured all patients with ISR treated in the four studies: 220 patients with ISR who received BVS, 249 who received a DCB, and 249 who received an everolimus-eluting stent.
There was no difference in the risk of all-cause or cardiac mortality between the three treatment groups. Overall, the rate of target lesion revascularization was 14.1% in the BVS group and 12.9% in the DCB-treated patients, a nonsignificant difference. In contrast, the target lesion revascularization rate was 5.2% in patients treated with DES. After adjustment for potential confounders, there was no significant difference in target lesion revascularization between BVS- and DCB-treated patients at 3 years, but BVS were associated with roughly a threefold higher risk of target lesion revascularization compared with DES.
The rate of target vessel revascularization was also significantly lower in patients treated with the everolimus-eluting stent compared with BVS, a difference that persisted after statistical adjustment (HR 1.85; 95% CI 1.02-3.34).
In-stent restenosis still remains a major clinical problem. Fernando Alfonso
In a landmark analysis focused on events beyond 1 year, clinical outcomes were similar between the BVS- and DCB-treated groups. The need for target lesion revascularization, however, was significantly higher with BVS compared with the everolimus-eluting stent (5.5% vs 2.0%; P = 0.035). After adjustment, BVS was associated with a threefold higher rate of clinically driven target lesion revascularization compared with both DES and DCB.
“One could speculate that by leaving a foreign metal in the body you’d pay a price in the long term, so we performed the landmark analysis after the first year when the BVS should have disappeared,” said Alfonso. “After adjusting for potential confounders, the target lesion revascularization rate was still higher for BVS and also higher than [it was] with drug-coated balloons.”
Four patients (1.8%) treated with BVS had a very late definite/probable stent thrombosis, compared with one patient treated with DCB and no events in the DES group. Accordingly, this study suggests that, beyond the first year, DCBs are superior to BVS in patients with ISR regarding safety and efficacy, said Alfonso.
Figuring Out Best Approach for ISR
Ajay Kirtane, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY), study chair of AGENT IDE, said that one the challenges with ISR is that the lumen gets smaller and smaller with more devices and that treatment depends on the reasons for restenosis.
“If the first stent that was put in wasn't fully expanded, then the thinking shouldn't be: what's the next device to use?” he said. “The thinking has to be, A, why did that not happen properly the first time? And B, is there a way that I can maximize my luminal dimension to prevent it from happening again? Irrespective of what device you use, if you have an underexpanded stent, the outcomes are going to be bad. I feel like the therapy needs to be dictated by making a diagnosis first.”
Current-generation DES are so good that physicians might treat ISR by reflexively placing another DES, but that approach won’t be effective if the first stent is underexpanded, said Kirtane.
Whenever possible, try to avoid another metallic layer if the first one could not be properly implanted. Tommaso Gori
Interventional cardiologist Tommaso Gori, MD (University Medical Center, Mainz, Germany), said there are a number of factors specific to the lesion, patient, and procedure that influence the initial failure of DES, making it difficult to consider a one-size-fits-all treatment for ISR. While the rate of TLR was higher with DCBs in the present study, Gori said they are an appropriate choice if the mechanism of restenosis is neointimal proliferation in a stent that can’t be expanded further.
“In that case, it is of paramount importance to further attempt to dilate the original stent and avoid further increasing the stent footprint by implanting a new DES,” he told TCTMD. “Understanding the cause of failure and attempting to treat it is crucial. . . . Whenever possible, try to avoid another metallic layer if the first one could not be properly implanted.”
In terms of treatment, Alfonso said DCBs do offer some advantages.
“I would say that in Europe most people select the drug-coated balloon for most patients with in-stent stenosis, especially if there is a good reason for that, such as a [patient with] high bleeding risk,” he said, noting the duration of dual antiplatelet therapy can be shortened. Additionally, DCBs are good options in patients who already have multiple stents or if there is a risk of jailing a side branch. “Some people might prefer that if the patient comes with the first in-stent restenosis to select a drug-coated balloon first because in the event there is a subsequent failure, we can still use a drug-eluting stent for recurrent in-stent restenosis, but this is quite operator-dependent.”
Kirtane ultimately urged caution interpreting the comparison between DES and DCB, noting that target lesion revascularization was driven in large part by angiographic follow-up data between 6 to 9 months.
“That really dramatically changes what happens because with a stent you're going to get a much more appealing picture because there's another scaffold inside,” he said. “With the DCB, it's not going to necessarily look as good, but did the patient need to have another revascularization? Most studies have shown that if you don't do angiographic follow up, then often you leave it alone. To me, the question of whether DES or DCB should be used is one that needs to be tested in the context of no angiographic follow-up.”
He pointed out, however, that if a vessel with restenosis is large enough to accommodate another DES, the clinical outcomes with this strategy will be very tough to beat. “If the lumen is big, DES is going to be a pretty good, durable strategy over the long term,” he said. “It remains to be seen whether DCB has an advantage over that approach.”
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Cuesta J, Pérez-Vizcayno MJ, del Blanco BG, et al. Long-term results of bioresorbable vascular scaffolds in patients with in-stent restenosis: the RIBS VI study. JACC Cardiovasc Interv. 2024;17:1825-1836.
Disclosures
- Alfonso reports no relevant conflicts of interest.
- Kirtane reports institutional funding to Columbia University and/or the Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CathWorks, CSI, Philips, ReCor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, and Shockwave Medical. In addition to research grants, institutional funding includes fees paid to Columbia University and/or Cardiovascular Research Foundation for consulting and/or speaking engagements in which Kirtane controlled the content. He also reports receiving travel expenses/meals from Amgen, Medtronic, Biotronik, Boston Scientific, Abbott Vascular, CathWorks, Concept Medical, Edwards, CSI, Novartis, Philips, Abiomed, ReCor Medical, Chiesi, Zoll, Shockwave, and Regeneron.




Comments