DETOUR 2: Endovascular Procedure That Mimics Femoral Bypass Durable at 3 Years
The results in patients with long, complex lesions are better than or equal to those of surgery, but without the complications.
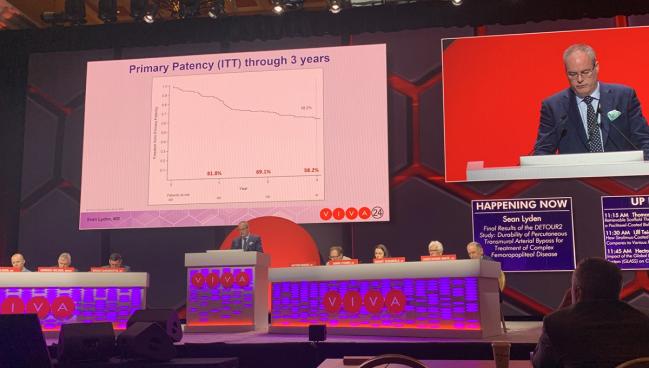
LAS VEGAS, NV—An endovascular approach for the treatment of long, complex superficial femoropopliteal lesions that incorporates surgical principles results in good patency through 3 years with no late venous issues or deep vein thrombosis (DVT) complications, according to final data from the DETOUR 2 trial.
In a presentation here last week at VIVA 2024, Sean P. Lyden, MD (Cleveland Clinic, OH), noted that while bypass works well for patients with long, complex disease, it is associated with extended hospital stays and readmissions, with up to 35% having wound complications. Many of these patients also do not fare well long term with less-invasive endovascular options, frequently needing repeat procedures.
DETOUR 2 investigated the clinical utility of a percutaneous transmural arterial bypass (PTAB) technique that mimics surgical bypass with stents that are routed through the femoral vein to restore blood flow. Many patients enrolled had already failed endovascular procedures or were not considered good candidates for open surgical bypass.
“The results, I would say, are better than or equal to surgical bypass without any of the open surgical cut down, the open surgical reconstruction, the wound issues, and the readmission rates, with either an outpatient or an overnight stay,” Lyden noted, adding that it “fills a unique role in our armamentarium.” Patency was 58.2% at 3 years, with a freedom from clinically driven TLR rate of 66.8%, and a bypass patency rate (flow versus no flow) of 84%.
The US Food and Drug Administration approved the Detour system (Endologix) in 2023 based on 12-month data from the DETOUR 2 study. The system consists of the Endocross spring-loaded dual guidewire delivery tool and Torus stent grafts in 5.5 mm, 6.0 mm, and 6.7 mm diameters. It can be used for femoropopliteal lesions ranging from 200 mm to 460 mm in length.
Following the presentation, moderator Brian DeRubertis, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY), questioned if this type of technique causes any lasting tissue damage that would be problematic down the road.
“As you go further out from this index procedure, especially one which would link a conduit to a vein, I can imagine having problems with venous insufficiency symptoms or venous changes, or things that are less consequential than a DVT, but still significant to the patient,” he added.
Lyden said the trial included venous clinical severity scores as well as Villalta scores, which are used to diagnose and categorize postthrombotic syndrome of the lower extremities, but saw no worsening from baseline through 3 years.
The first thing I tell them before I touch them is, ‘You're going to be coming back for life. Sean P. Lyden
“The one thing I can tell you is that you do have to let your vascular lab know that this goes through the vein,” he said. “Just like a central venous catheter or anything else you put through the vein, there will be a fibrin sheath.” Knowing the sheath is there is important to avoiding misdiagnoses of DVT because the new blood flow channel is essentially hiding within it, he added.
To TCTMD, Lyden said while the concept of PTAB is new, the learning curve for doing it is actually fairly quick, noting that one of his partners who was not involved in the DETOUR 2 trial is now doing the procedures in about an hour. He said the most important part of the procedure is good ultrasound guidance to access the vein. “That's the hardest part. There's nothing unique or difficult about the procedure,” Lyden noted.
Ehrin Armstrong, MD (Advanced Heart and Vein Center, Denver, CO), a panelist in a recap of the VIVA late-breaking trial sessions, said he has incorporated the procedure into his practice and found it to be “a useful additional therapy for people who've had multiple percutaneous coronary interventions and have failed a few times from a standard endovascular approach, especially if they don't have good native vein.”
Commercialization and Follow-up
DETOUR 2 enrolled 202 patients (mean age 68.9 years; 26% female) with Rutherford class 3 to 5 disease at 36 sites in the United States and Europe. More than 85% of patients had hypertension, nearly 70% had hyperlipidemia, one-third had diabetes, 46% had CAD, about 88% had hypertension, and 91% had a smoking history.
The majority of patients had chronic total occlusions and diffuse stenosis. The mean lesion length was 327 mm in the femoropopliteal segment, with severe calcification in 70%. Prior to the PTAB procedure, 76% of patients had undergone percutaneous transluminal angioplasty (PTA). For inclusion in DETOUR 2, patients were required to have < 50% stenosis in a popliteal artery distal to the landing zone with at least one patent tibial artery extending to the foot and a patent femoral vein ≥ 10 mm in diameter or a duplicate femoral vein.
The mean total procedure time was 181 minutes, and the average length of stay was 1 day. One patient experienced a procedure-related infection through 30 days. Clinical success, defined as limb ischemia improvement as assessed by Rutherford Clinical Classification at 3 years, was 98.7%.
The rate of major index limb amputation was 1.5% at 3 years, with an all-cause mortality rate of 8.9%. The majority of patients (96%) were free of a DVT event at 3 years, which was unchanged from the 2-year follow-up.
Lyden noted that the commercialization of the system has led to some tweaks to make the Endocross device simpler to operate and that real-world commercial experiences are being tracked in a postmarket study.
To TCTMD, he said other than one incident during the DETOUR 2 trial, there have been no cases where surgical conversion was needed, clarifying that the single case was in a patient who didn’t meet all the trial’s enrollment criteria.
Lyden said having the system as an option for management helps him to have discussions with his PAD patients about the reduced likelihood of needing the same type of repeat endovascular procedures that many of them have been through again and again. However, he said, part of that discussion includes the importance of repeat follow-up and making patients understand that the PTAB procedure itself isn’t a cure for their vascular disease or a replacement for behavior modifications like quitting smoking and managing comorbid conditions.
“Many of my patients want an easy, simple fix, and I make it clear that that’s not what is going to happen,” he said. “The first thing I tell them before I touch them is, ‘You're going to be coming back for life. We're going to treat you until either I die, or you die, . . . but you need to come back forever. If we find a problem, we can salvage it before we let it fail.’”
Lyden added that the postmarket study will provide more information about the types of reinterventions that these patients may need over the long term and how best to manage them.
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Lyden SP. Final results of the DETOUR2 study: durability of percutaneous transmural arterial bypass for treatment of complex femoropopliteal disease. Presented at: VIVA 2024. November 5, 2024. Las Vegas, NV.
Disclosures
- Lyden reports consulting for Abbott, Boston Scientific, Endologix, Intact Vascular, Medtronic, Penumbra, Philips, and Shockwave Medical.





Comments