Direct Delivery of Antibiotics to CV Device Pocket Infections Appears Promising
The approach can be considered in “super-selected” patients, but removal of all hardware remains the go-to, Anne Curtis says.
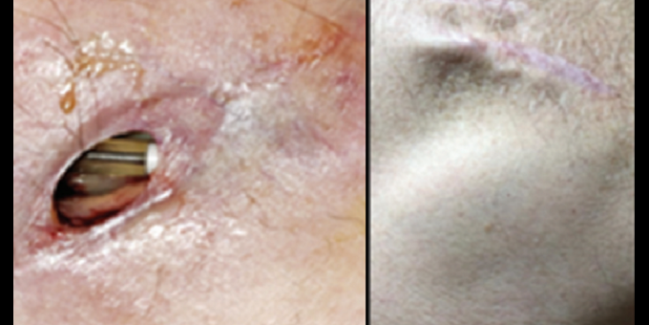
Pacemaker electrodes visible through the dehisced wound (left) and healed device pocket at 1-year follow-up (right). Photo Credit: J Am Coll Cardiol. Central Illustration (adapted).
When device/lead extraction is not indicated or desired for an infection related to a cardiovascular implantable electronic device (CIED), direct delivery of high-dose antibiotics to the infected pocket may be an option, a preliminary study suggests.
The technique, developed by Moris Topaz, MD, PhD (Sourasky Tel Aviv Medical Center, Tel Aviv, and Hillel Yaffe Medical Center, Hadera, Israel), in 2007, involves delivery of a continuous, in situ-targeted, ultrahigh concentration of antibiotics (CITA) into the infected device pocket via an indwelling catheter.
Over a 14-year experience, reported in a study published online ahead of the January 17, 2023, issue of the Journal of the American College of Cardiology, CITA avoided the need for extraction—the standard of care—in 90.8% of patients eligible for complete removal of the CIED and all leads. Although extraction was associated with higher cure rates, it also came with a greater risk of serious complications.
The results are reminiscent of the early days of angioplasty for CAD, when the less-invasive approach avoided the need for surgery but came with a higher rate of recurrence, senior author Sami Viskin, MD (Sourasky Tel Aviv Medical Center and Tel Aviv University), told TCTMD via email.
“The most important clinical implication is that CITA, as performed by Dr. Topaz and as described in the study, is a legitimate alternative to complete lead extraction, particularly for patients who are at particularly high risk for extraction because of advanced age, fragility, and a high number of leads implanted for longer periods of time,” he said.
“All patients in need for extraction can now make an informed choice about the treatment they wish to undergo,” said Viskin. “The new procedure is, by far, less invasive (and therefore less risky) than lead extraction.”
CITA failed in 15% of patients, but “we would like to emphasize that extraction is always a possibility if CITA fails,” Topaz, one of the lead authors along with Ehud Chorin, MD, PhD (Sourasky Tel Aviv Medical Center and Tel Aviv University), noted via email. “CITA should be considered as one more treatment option to be considered for patients with infection localized to the subcutaneous pocket.”
Preventing Potentially Dangerous Extractions
Either local or systemic infections are serious complications in patients with CIEDs, including implantable cardioverter-defibrillators (ICDs), pacemakers, or cardiac resynchronization therapy (CRT) devices. The guideline-recommended approach to treating these infections is removal of the device and any leads, but extraction is risky, possibly resulting in venous tearing, cardiac perforation, tricuspid regurgitation, and even death.
“Lead extraction remains [one of] the most risky procedures performed by cardiac electrophysiologists,” Viskin noted.
Topaz developed CITA, which is performed along with wound revision and capsular debridement, to as an alternative to extraction in select patients, and the current study reflects the results obtained in patients treated between 2007 and 2021. The analysis included 80 patients (mean age 70 years; 21.2% women) with pocket infections; most (81%) were eligible for extraction but opted for CITA, and the rest either had prohibitive operative risk or had questionable indications for extraction.
The CITA program was not for everyone, as it excluded patients with signs of systemic infection, fever, positive blood cultures, lead vegetations, or Staphylococcus aureus growth in pocket cultures at the time of recruitment.
In the overall cohort, CITA resulted in cure—meaning patients were free from infection throughout a median follow-up of 3 years—in 85% of cases. The failed cases included three patients who died, six who ultimately underwent extraction, and three who developed a chronic fistula to the CIED pocket following repeated CITA failure.
The investigators then compared the 65 patients who were eligible for extraction but who underwent CITA treatment with 81 similar patients who underwent device/lead extraction as the initial approach. The cure rate was higher with extraction (96.2% vs 84.6%; P = 0.027), but so were serious complications (14.8% vs 1.5%; P = 0.005), which included stroke, the need for a thoracotomy, urgent blood transfusions, and severe valvular damage.
All-cause mortality was not significantly different between the CITA and extraction groups at either 1 month (zero vs 3.7%) or 1 year (12.3% vs 13.6%; P = NS for both).
Use in Selected Patients
Anne Curtis, MD, who wrote an accompanying editorial with Aamir Ahmed, MD (both from the University at Buffalo, NY), pointed out to TCTMD that the recommended approach to treating a CIED-related infection, whether localized to the pocket or spread elsewhere, is removal of all hardware because it’s been shown to be difficult to eradicate bacteria without doing so.
Extraction, however, can be associated with poor outcomes, including catastrophic bleeding and death, she stressed. “You do it when you have to, but you’d like not to have to.”
In cases where the infection seems to be confined to the pocket, “there is sort of a desire to hope you can just . . . open up the pocket, sort of clean out the puss, and hope you’re going to be okay,” Curtis said. “And most of the time, you’re not. It’s very rare that people get away with that.”
The CITA approach detailed here seems to be able to manage some of these types of infections, she said, noting that certain patients, such as those with difficult-to-treat bacterial infections, were excluded.
Nevertheless, Curtis said, “In super-selected patients—not when you have a systemic infection, not when you have Staph aureus—it’s something that can be considered.” Most patients probably still should be treated with extraction, she added, but CITA could be an option in patients with a very high surgical risk and in those who are very old and frail, for example. “In very carefully selected patients, I could see doing it now.”
Topaz acknowledged that more research is needed to answer some remaining questions about CITA. “Additional, wider-scale research should substantiate our work and evaluate additional antibiotics and infecting agents; a more definitive length of treatment needs to be established,” he said. “The importance of early administration of CITA and objective and accurate techniques to determine the complete eradication of infection are some of the research that still needs to be conducted.”
Viskin pointed to promising early results but the limited experience with CITA, and said, “It is certainly a nice beginning . . . to be continued.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Topaz M, Chorin E, Schwartz AL, et al. Regional antibiotic delivery for implanted cardiovascular electronic device infections. J Am Coll Cardiol. 2023;81:119-133.
Curtis AB, Ahmed A. Treatment of localized implantable cardiac device pocket infections. J Am Coll Cardiol. 2023;81:134-135.
Disclosures
- Topaz reports being the developer and patent holder of TopClosure Tension Relief and Vcare α and MeCare systems, and heading and holding shares in IVT Medical, the manufacturer of these devices.
- Chorin and Viskin report no relevant conflicts of interest.
- Curtis reports having served on the advisory board for Medtronic, Abbott, Janssen Pharmaceuticals, Sanofi, Milestone Pharmaceuticals, and Eagle Pharmaceuticals; and having received honoraria for speaking from Medtronic and Abbott.
- Ahmed reports having served as a clinical consultant for Medtronic.



Comments