DOACs Reduce Stroke, Increase Bleeding in ICH Survivors With AF
PRESTIGE-AF is the first of the larger trials in this scenario to report results. More data are needed to help guide decisions.

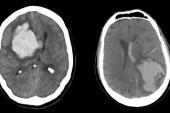
Among patients with atrial fibrillation (AF) who have survived an intracerebral hemorrhage (ICH), taking a direct oral anticoagulant (DOAC) markedly lowers the risk ischemic stroke but at the cost of more recurrent hemorrhages, the PRESTIGE-AF trial shows.
Overall, however, the net clinical benefit—based on an endpoint incorporating all stroke and systemic embolism, MI, cardiovascular death, and major bleeding—tends to lean toward giving the DOAC versus not treating with an anticoagulant (adjusted HR 0.69; 95% CI 0.33-1.40), Roland Veltkamp, MD (Imperial College London, England), and colleagues report in a paper published online recently in the Lancet.
“Stroke neurology traditionally focuses on prevention of strokes,” said Veltkamp, who presented the results last month at the International Stroke Conference. “However, we have to take other endpoints into account. Mortality and other bleedings on the one hand, and on the other hand, other ischemic outcomes like systemic embolism.”
Most of the efficacy endpoints in this trial favored DOAC therapy, though not significantly, and “taking all of this into account, I think that the data show more benefit than harm for direct oral anticoagulants,” Veltkamp told TCTMD.
Determining whether an ischemic stroke or a recurrent hemorrhage has a greater impact on the patient is tricky, he added. “It’s a question that at this point in time, we cannot answer.”
Commenting on the findings for TCTMD, Kevin Sheth, MD (Yale School of Medicine, New Haven, CT), noted that PRESTIGE-AF is the first in a series of ongoing trials exploring this question that have reported results. Though there was an increase in recurrent ICH in the DOAC arm, the reduction in ischemic stroke was “quite dramatic,” he said. “That’s very encouraging.”
But there is a need for data from the other ongoing trials—like ENRICH-AF and ASPIRE, which include larger numbers of patients—to help answer the question about how to manage ICH survivors with AF, Sheth indicated. “It’s critically important for these brain hemorrhage survivors that have been left out of all of the anticoagulation trials to be entered into one of these studies, especially in the United States.”
An Unmet Need
Veltkamp described stroke prevention in ICH survivors as “an important unmet need.” These patients have a high risk of recurrent ICH as well as an increased risk of ischemic events, including stroke. A particularly vulnerable subset are those who also have AF (about 25% of ICH survivors), making it difficult to balance the need for protection against ischemic stroke and the need to keep bleeding risks low.
In a prior meta-analysis of observational studies, Veltkamp’s team showed that anticoagulation with a vitamin K antagonist reduced the risk of ischemic stroke compared with no anticoagulation without significantly increasing the risk of recurrent ICH. “We knew, however, that these observational data were prone to selection bias and confounding by indication,” Veltkamp said. “There was a clear need for randomized controlled trials.”
Subsequently, two pilot RCTs—SoSTART and APACHE-AF—showed that the net clinical benefit of anticoagulation in this scenario was unclear; a meta-analysis of those and two other trials provided similar results.
PRESTIGE-AF, a phase IIIb trial, was designed to provide more clarity. The trial included 319 patients (median age 79 years; 35% women) who had a spontaneous ICH within the last 12 months and had AF with an indication for oral anticoagulation; they were enrolled at 63 sites in six European countries.
Investigators randomized patients to DOAC therapy or no anticoagulation, with a median follow-up of 1.4 years. The most common DOAC chosen by local physicians was apixaban (53.8%). In the control group, 67% of patients received no antithrombotic, 30% aspirin, and 2% clopidogrel.
This trial was really important because it was the largest published trial to date that starts to give us high-quality scientific estimates on these endpoints. Kevin Sheth
In a superiority analysis, DOACs significantly reduced the risk of ischemic stroke versus no anticoagulation—one versus 20 events (adjusted HR 0.05; 95% CI 0.01-0.36).
DOACs failed, however, to meet the standard for noninferiority compared with no anticoagulation when it came to the risk of recurrent ICH. There were 11 recurrent events in the DOAC arm and one in the control arm (HR 10.89; 95% CI 1.95-60.72).
In terms of secondary outcomes, there were no differences between groups in all-cause mortality, CV mortality, MACE, and all stroke or systemic embolism, though there were numerically fewer events for each of these endpoints in the DOAC arm. Risks of any major hemorrhage and any intracranial hemorrhage were significantly higher with DOACs.
The researchers determined that the number needed to treat to prevent one ischemic stroke per year was 13, lower than the number needed to harm (to cause one more ICH per year) of 24.
Personalized Approach Should Be Explored
Sheth pointed out that ICH survivors often receive poor secondary prevention. “They sort of get caught in the black hole between cardiologists and neurologists and primary care,” he said. “In terms of their risk factors for things like A-fib, I’m telling you, a lot of them, you can see this even in the trials, they fall through the cracks.”
Management of these patients varies widely out in practice, he said. As in PRESTIGE-AF, some patients get no antithrombotic therapy, while the rest get a mix of aspirin, other antiplatelets, or left atrial appendage occlusion (LAAO).
In that landscape, “this trial was really important because it was the largest published trial to date that starts to give us high-quality scientific estimates on these endpoints,” Sheth said. “And they’ll really be reinforced, I think, with the even larger trials that are about to be published.”
Personalization of medicine I think is going to be the future for such patients with opposing risks. Roland Veltkamp
The wrong conclusion to take away from PRESTIGE-AF, he stressed, “would be to say that they had a higher rate of hemorrhages and therefore they all need a Watchman device.” Though LAAO might be an option in select patients, he said, “it needs to be rigorously studied in trials.” He pointed to a French trial called A3ICH randomizing patients to three arms—no anticoagulation, DOAC, and LAAO—as an example.
Sheth reiterated that getting patients to enroll in the ongoing trials is critical to work out the best ways to manage this scenario. “I would say they absolutely need to be entered into one of these large, randomized studies so we can have point estimates that are precise, not just about the right antithrombotic therapy, but also so that we can understand where devices like the Watchman may play a role,” he said.
Veltkamp said LAAO is “probably a good solution for selected patients,” though he added that “this is the subject of ongoing clinical trials and we have to see whether in this vulnerable patient population, left atrial appendage occlusion is as safe as it is in other patients.”
He said more data are needed in this area, and that there is a need for tailored treatment approaches. “I personally think that we need markers to distinguish patients—personalization of medicine I think is going to be the future for such patients with opposing risks,” Veltkamp suggested.
But it will take time to identify genetic and imaging markers, and other factors, that can be used to determine who will or will not benefit from anticoagulation, Veltkamp said.
PRESTIGE-AF is “a significant step forward in the story,” he added, “but the story is not completely told.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Veltkamp R, Korompoki E, Harvey KH, et al. Direct oral anticoagulants versus no anticoagulation for the prevention of stroke in survivors of intracerebral haemorrhage with atrial fibrillation (PRESTIGE-AF): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2025;Epub ahead of print.
Disclosures
- The study was sponsored by Imperial College London and was funded by the European Commission as part of the Horizon 2020 research and innovation program.
- Veltkamp reports receiving research support from Bayer, BMS-Pfizer, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, and Biogen; receiving honoraria for consultancies and lectures from AstraZeneca, Bayer, BMS-Pfizer, Javelin, and Portola; and being an investigator of the Imperial BRC.



Comments