Embolic Protection May Help During TAVI, but Stroke Impact Likely Small
In a new TVT Registry analysis, the relative reduction in risk of disabling stroke was lower than that seen in PROTECTED TAVR.
NEW YORK, NY—Use of a cerebral embolic protection device (EPD) during TAVI may prevent disabling strokes, but any effect appears to be modest, according to an updated analysis of the Society of Thoracic Surgeons/American College of Cardiology TVT Registry.
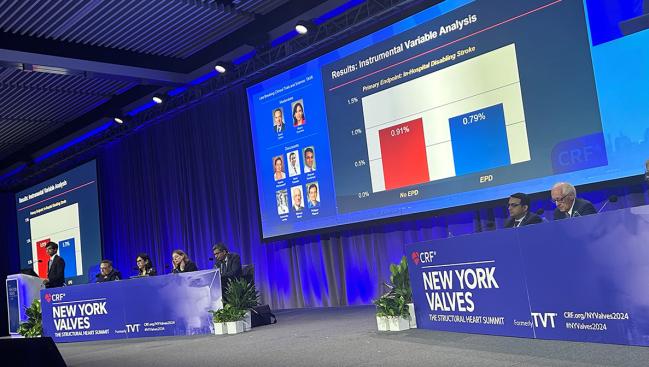
Disabling stroke—assessed using in-hospital death and discharge to somewhere other than home after stroke as a proxy—occurred in 0.79% of patients who underwent TAVI with a protection device in place and 0.91% of those who underwent the procedure without embolic protection, Neel Butala, MD (Rocky Mountain Regional VA Medical Center and the University of Colorado School of Medicine, Aurora), reported here at New York Valves 2024, previously known as the TVT meeting.
The difference was only borderline significant in the primary instrumental variable analysis (RR 0.87; 95% CI 0.73-1.00), which Butala said can be used to support causal inference. Of note, the relative reduction in risk of disabling stroke was much smaller than what was observed in the PROTECTED TAVR trial (13% vs 62%).
Nonetheless, “these findings are congruent with those from the PROTECTED TAVR trial and suggest that reduction in disabling stroke from EPD use is real,” Butala said. He added, however, that “the effect size may be relatively small in all-comer patients.”
The results were published simultaneously online in Circulation: Cardiovascular Interventions.
Dharam Kumbhani, MD (UT Southwestern Medical Center, Dallas, TX), wrote an accompanying editorial with Frederick Welt, MD (University of Utah Health Sciences Center, Salt Lake City). Commenting on the findings for TCTMD, Kumbhani indicated that interpretation of the results should be influenced by the method used to ascertain disabling stroke.
That limitation aside, “it seems very plausible based on what we’ve seen with PROTECTED TAVR and this analysis that there is maybe a small effect that embolic protection has in preventing larger strokes,” Kumbhani said, adding that “how we choose patients for this is still, I think, unclear.”
No Impact on Nondisabling Strokes
Stroke remains a concern after TAVI, and cerebral embolic protection devices have been developed with the aim of reducing that risk. There is a lack of definitive evidence that they reduce clinical strokes, however. A prior analysis of the TVT Registry from Butala’s group didn’t show an association between EPD use and in-hospital stroke in the primary instrumental variable analysis. And in PROTECTED TAVR, the largest trial to date, patients randomized to receive protection during the procedure did not have a significantly reduced risk of periprocedural stroke, the primary endpoint, although there was reduction in disabling stroke, an exploratory secondary endpoint.
To further explore the potential impact of embolic protection during TAVI, Butala et al again turned to the STS/ACC TVT Registry. This new analysis included 414,649 patients (mean age about 79 years; 43% women) who underwent transfemoral TAVI at 808 US sites between January 2018 and June 2023.
Overall, 12.9% of patients underwent the procedure with the Sentinel embolic protection device (Boston Scientific), the only EPD commercially available during the study period, in place. That proportion increased from 5% in the first quarter of 2018 to 15% in the third quarter of 2020, but then eventually declined to 12% in the fourth quarter of 2022 following release of the PROTECTED TAVR results. “This indicates that as a community we respond to TAVR trial results and change our practice,” Butala said.
There was wide variation in use, however, with 6.3% of centers using protection in more than half of their TAVIs and 39.8% using it in none of their cases.
The investigators’ prior analysis used all strokes as the primary outcome, but this updated analysis used disabling stroke. The TVT Registry doesn’t directly capture stroke severity, so the researchers developed a proxy measure based on discharge location. Defining a disabling stroke as one that resulted in in-hospital death or discharge to a location other than home was shown to have a sensitivity of 73% and a specificity of 90% for identifying the event in a separate pooled analysis of large TAVI trials conducted after 2017.
Although the difference in risk of disabling stroke was only borderline significant in favor of EPD use in the primary instrumental variable analysis, the advantage was significant in a secondary propensity-matched analysis (OR 0.79; 95% CI 0.70-0.90).
Neither overall in-hospital stroke nor nondisabling stroke was significantly influenced by EPD use in the instrumental variable analysis, but embolic protection was associated with a lower likelihood of any stroke after propensity score matching (OR 0.84; 95% CI 0.76-0.94).
A prespecified subgroup analysis indicated that protection might have a greater benefit in patients with a history of stroke (about 10% of the TAVI population), with an absolute risk difference favoring protection of 0.65% in patients with such a history and 0.06% among those without prior stroke.
A Modest Effect
If indeed there is a significant benefit of embolic protection for reducing disabling strokes, the effect is likely to be modest, the researchers say, calculating a number needed to treat of 833 based on the event rates observed here.
That modest benefit supports equipoise for continuation of the BHF PROTECT-TAVI trial ongoing on in the United Kingdom, though it also suggests “that a clinical benefit is unlikely to be found with its intended sample size,” the investigators write. Even the planned pooled analysis of that trial and PROTECTED TAVR will probably be underpowered, they add, estimating that an RCT would have to enroll about 97,000 patients to detect a difference in any in-hospital stroke with 80% power and about 132,000 patients to detect a difference in disabling stroke.
Discussing the results after Butala’s presentation, Alexandra Lansky, MD (Yale University School of Medicine, New Haven, CT), said the results—from the largest series looking at stroke and the impact of EPDs during TAVI—complement what’s already known from prior research and are consistent with those from PROTECTED TAVR, albeit with a smaller effect size.
She highlighted the results in the subgroup with prior stroke, saying, “We’ve been trying to find a cohort of patients that would benefit more from EPD, and I think you kind of nailed it. The sample size here is large enough that we can say that patients with prior stroke are probably the ones that we want to target.”
Lansky had some caveats, however. The lower relative risk difference observed in this study versus PROTECTED TAVR is likely related to how the events were ascertained, she said, calling the proxy that was used “imperfect.” That probably led to underestimation of event rates and the treatment effect, she added.
Lansky asked Butala where he stands in terms of EPD use, and he said, “I personally think the subgroup analyses here are pretty revealing. I think there’s strong evidence suggesting we should use it in patients with prior stroke.” Further research is needed, he added, for other groups in which there was a hint of greater benefit, including in those undergoing valve-in-valve procedures.
Overall, though, the results indicate that “there may be a benefit,” Butala said, acknowledging that for the average patient, “it may be a very small benefit.” In that context, other factors, like the time and cost that EPD use adds to the TAVI procedure, come into play.
Kumbhani said this study “certainly does not provide justification for using it in all patients,” but he stressed that there’s no validated way to identify which patients are likely to have a stroke after TAVI. He said his group had been using EPDs selectively up until a couple of years ago, but then after there were a few strokes in patients that didn’t have clear high-risk features, he and his colleagues decided to use an EPD during all TAVI procedures moving forward.
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Butala NM, Kapadia S, Secemsky EA, et al. Impact of cerebral embolic protection devices on disabling stroke after transcatheter aortic valve replacement: updated results from the STS/ACC TVT Registry. Circ Cardiovasc Interv. 2024;Epub ahead of print.
Butala NM, Kapadia S, Yeh RW, Cohen DJ. Use of discharge disposition to determine stroke severity after TAVR. Circ Cardiovasc Interv. 2024;Epub ahead of print.
Kumbhani DJ, Welt F. Supplementing randomized trial data to answer a real world question: discharge to home status as a heuristic for stroke severity after transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2024;Epub ahead of print.
Disclosures
- The study was funded in part by an investigator-initiated grant from Boston Scientific.
- Butala reports support from the Boettcher Foundation Webb-Waring Biomedical Research grant; consulting fees from Shockwave Medical; and consulting fees and ownership interest from HiLabs.
- Lansky reports consulting or speaking fees or honoraria from Abiomed, Boston Scientific, Cordis, and MedAlliance and grant support/research contracts from Abbott, Abiomed, and Sinomed.
- Kumbhani and Welt report no relevant conflicts of interest.





Comments