Embolic Protection Snags Debris in Nearly All Lower-Risk TAVI Patients
The field awaits the results of PROTECTED TAVR to see whether the trial can deliver proof that the device reduces stroke risk.
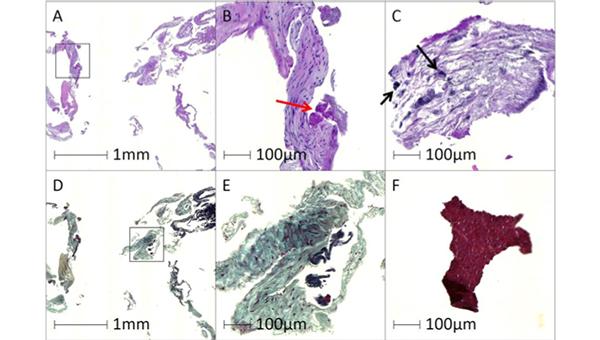
In a small, prospective study of low-to-intermediate-risk patients undergoing TAVI, the Sentinel cerebral protection system (Boston Scientific) captured debris in all cases, with the features of the particles similar to what has been seen in higher-risk cohorts, researchers report.
The debris included bits of arterial wall in 98% of cases, acute thrombus in 96%, valve tissue in 71%, calcification in 55%, and foreign materials in 43%, according to researchers led by Rika Kawakami, MD (CVPath Institute, Gaithersburg, MD). Pieces of myocardial tissue, organizing thrombus, and necrotic core were less common.
“Overall, the results of the present study are align[ed] with those of previous studies conducted in the high-surgical-risk population, undergoing TAVR with the Sentinel cerebral protection system, regarding the capture rate of debris, observed tissue types, and size/distributions of debris,” Kawakami et al write in a research letter recently published online in Circulation: Cardiovascular Interventions.
Of note, more than three-quarters of particles were smaller than 500 μm, which is probably not big enough to cause a major stroke, senior author Aloke Finn, MD (CVPath Institute), told TCTMD. More of interest, he said, are the 5% of particles that were 1,000 μm or larger (found in 67% of cases), “which is supposedly big enough to lodge in a vessel and cause significant flow obstruction.”
Overall, the study “continues to support the potential use of the embolic protection devices in lowering stroke risk during the TAVR procedure,” Finn said. “It’s not definitive, obviously, but it’s suggestive that these embolic protection devices are helpful.”
Stroke Concerns
Stroke remains a serious concern after TAVI, despite declining rates over time. Even among the low-risk patients enrolled in the Evolut Low Risk Trial, the 30-day stroke rate was 3.4%.
Though prior studies have shown that deploying the Sentinel system captures debris in nearly all patients undergoing TAVI and have suggested that doing so may reduce stroke in intermediate- or high-risk cohorts, definitive evidence of a reduction in events remains elusive. Moreover, use of embolic protection with the Sentinel had not been evaluated in lower-risk patients.
It’s not definitive, obviously, but it’s suggestive that these embolic protection devices are helpful. Aloke Finn
The prospective SENTINEL-LIR study, conducted at four US centers, was designed to do just that. It included 50 patients (mean age 76 years; 45% women) with severe symptomatic aortic stenosis who underwent transfemoral TAVI with planned use of the Sentinel system between March and August 2020. All had an STS PROM score below 4% (median 1.7%), with most (86%) deemed by a heart team to have a low surgical risk; the remaining 14% were classified as intermediate risk.
For the procedure, 55% of patients received a CoreValve Evolut Pro valve (Medtronic) and the rest were treated with a Sapien 3 valve (Edwards Lifesciences). Predilatation and postdilatation were used in 43% and 18% of cases, respectively, and these rates didn’t differ by transcatheter valve type. Both the proximal and distal filters of the Sentinel system were successfully placed in all patients.
Within 30 days of TAVI, one patient died from an MI and two (4%) had a stroke.
The Sentinel filters captured at least some debris in all cases. Most of the particles (78%) were between 150 and 500 μm in size, whereas about 5% were 1,000 μm or larger. These larger particles were found in two-thirds of patients.
Calcified particles were more frequently found in patients who had predilatation performed (71% vs 43%; P = 0.046), although predilatation and postdilatation were not related to particle numbers or sizes. Transcatheter valve type was not associated with any of the debris characteristics.
Looking to PROTECTED TAVR for Definitive Answers
Commenting on the findings for TCTMD, Steven Yakubov, MD (OhioHealth Riverside Methodist Hospital, Columbus), said the types of debris captured were “virtually identical” to what’s been seen in higher-risk populations, confirming what he would have expected.
“This gives more evidence that debris during transcatheter valve replacement occurs in low-risk, intermediate-risk, and high-risk patients. It’s indiscriminate. And Sentinel is very useful in capturing the debris,” he said.
Yakubov called himself “a believer in embolic protection from an anecdote-based, experiential viewpoint,” saying that “what I’ve seen in my own practice is that Sentinel has helped reduced substantially . . . the incidence of stroke.” The inability for the trials done thus far to definitively prove a reduction in stroke by using embolic protection has been disappointing, Yakubov said, pointing to the low numbers of patients in the prior studies as one potential reason to explain the results.
Because major stroke is a low-frequency event, it will take a study with a large number of patients to demonstrate an impact from using the Sentinel device, he said. “I am hoping that PROTECTED TAVR”—a study of 3,000 patients—“is able to do that.”
In the absence of definitive trial evidence, use of the Sentinel system varies across centers. Yakubov said embolic protection is used in every TAVI case at his center, with the exception of patients with anatomical exclusions (a group he estimated makes up less than 5% of cases).
“If it were my preference, I would like to have embolic protection on every patient,” he said.
Both the lack of a proven reduction in stroke in a randomized trial and the added cost of the protection devices play into the spotty uptake of the approach, Yakubov and Finn indicated.
“Payment doesn’t meet the cost of the embolic protection device, and many sites struggle to make TAVR a break-even proposition financially for the institution,” Yakubov said, noting that the add-on payment implemented for the Sentinel system by the US Centers for Medicare & Medicaid Services back in 2018 doesn’t fully cover the cost.
Acknowledging the fact that a reduction in stroke has not been conclusively shown in a randomized trial, as well as the added cost of the Sentinel device, Finn said lower-risk patients are likely the group that would derive the most benefit from protection because they’re generally younger and have more years of life ahead of them.
“These are the target population where these devices should be used without hesitation,” Finn said. “I don’t think there’s a big risk to using these devices. It doesn’t add a lot of time to the procedure. It’s very simple to deploy. . . . Preventing a stroke, even if it’s one or two in a hundred patients, might be worth it because of the extra cost of the hospital stay, the morbidity, and the mortality [associated with] stroke.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Kawakami R, Gada H, Rinaldi MJ, et al. Characterization of cerebral embolic capture using the SENTINEL device during transcatheter aortic valve implantation in low to intermediate-risk patients: the SENTINEL-LIR study. Circ Cardiovasc Interv. 2022;15:e011358.
Disclosures
- The study was funded by Boston Scientific.
- Kawakami reports having received research grants from the Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad.
- Finn reports having received honoraria from Abbott Vascular, Biosensors, Boston Scientific, Celonova, Cook Medical, CSI, Lutonix Bard, Sinomed, and Terumo Corporation and serving as a consultant to Amgen, Abbott Vascular, Boston Scientific, Celonova, Cook Medical, Lutonix Bard, Sinomed, Surmodics, Terumo Corporation, W.L. Gore, and Xeltis.
- Yakubov reports no relevant conflicts of interest.





Comments