EVEREST: MitraClip Safe, Effective for Mitral Valve Repair
SAN FRANCISCO, Calif.—High-risk patients with mitral regurgitation who were implanted with the Evalve MitraClip derived clinical benefit at 12 months, according to results from the EVEREST trial and registries.
According to results from the EVEREST II high-risk registry, left ventricular end diastolic volume decreased from 172 mL to 140 mL at 12 months (P<.0001), coupled with a decrease in LV end systolic volume (from 82 mL at baseline to 73 mL) at 12 months for patients treated with the MitraClip. There was an increase in freedom from death in the MitraClip group vs. surgical controls at 30 days (P=.037) (see Figure 1), and a 45% decrease in rehospitalizations for congestive HF.
“The absence of intraprocedural mortality, as well as the stability of the patients during the procedure, is remarkable,” said Ted Feldman, MD, of Evanston Hospital in Illinois. “They are much more stable than aortic valve intervention patients and actually are even a lot more stable than simple PCI patients, and that has been a really great part of the procedure.”
MitraClip for functional mitral regurgitation
Researchers formed an additional cohort of 23 patients from the EVEREST trial program who had functional mitral regurgitation. According to James B. Hermiller Jr., MD, of the St. Vincent Heart Center of Indiana in Indianapolis, the implantation procedure for the MitraClip was completed in 19 of the 23 patients. According to the results, there were improvements in LV end-diastolic (P<.04) and systolic (P<.03) dimensions. No change in ejection fraction was reported at 12 months.
“These are encouraging data in patients with functional mitral regurgitation with the MitraClip and suggest that these patients benefit substantially in terms of functional class, mitral regurgitation reduction and favorable ventricular remodeling,” Hermiller said.
Patrick L. Whitlow, MD, director of interventional cardiology at the Cleveland Clinic, presented data from the EVEREST U.S. high-risk registry that included 78 patients who were not randomized in the EVEREST II trial. The registry had a primary safety endpoint of 30-day mortality and a combined efficacy endpoint that included reduction of mitral regurgitation, freedom from death, and improvement in NYHA functional class.
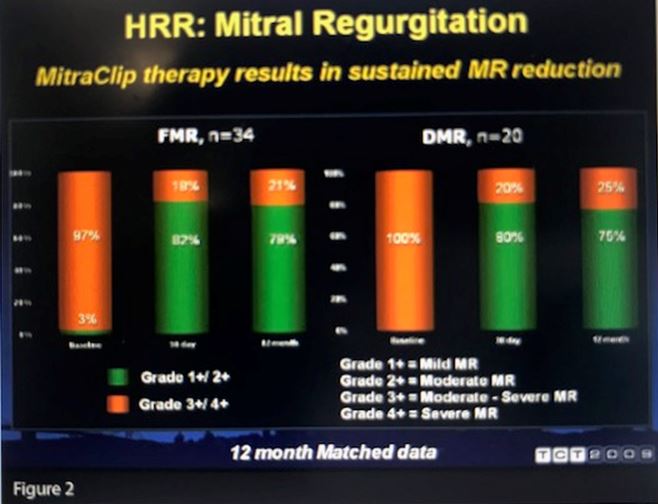
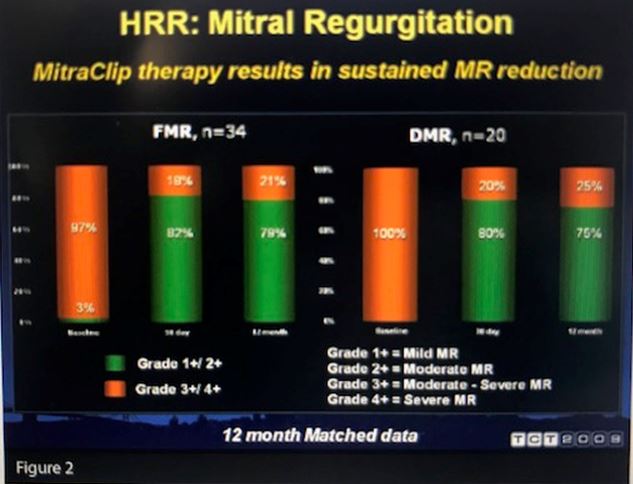
Whitlow reported a 96% implantation success rate. Actual 30-day mortality was lower than the predicted rate (7.7% vs. 18.2%, P=.006), although 20 patients had major adverse events at 30 days, including six deaths. At 12 months, patients implanted with the MitraClip had reductions in mitral regurgitation, improved NYHA functional class, as well as reverse LV remodeling, improved forward stroke volume and a reduction in diastolic (P<.0001) and systolic (P=.008) septal-annular dimension (see Figure 2).
“These data demonstrate that we can put the MitraClip in these high-risk surgical patients very safely and that the patients do derive clinical benefit,” Whitlow concluded.
MitraClip following CE Mark approval
Francesco Maisano, MD, of the San Raffaele Hospital in Milan, Italy, noted that there was a large population at risk in Europe and an unmet clinical need for mitral valve repair using the MitraClip. He cited several challenges that also should be considered: lack of clinical evidence, cost-effectiveness data, reimbursement strategies, training and known efficacy of surgical repair for degenerative mitral regurgitation. The European experience with the MitraClip is currently limited, but Maisano predicted that would likely change in the future as training increases and reimbursement strategies are resolved.
“Evalve has opened a new era of transcatheter mitral valve repair, and the MitraClip offers an alternative to patients with contraindications for surgery,” Maisano said. “In Europe, we have shown that patient selection criteria have gone beyond the EVEREST trial requirements both in European practice and in the REALISM registry.”
Disclosures
- Drs. Feldman and Hermiller report receiving research support from Evalve
- Dr. Whitlow reports receiving research grant support from Evalve and being a stock shareholder in Medtronic.
- Dr. Maisano reports receiving research grant support from St. Jude Medical and speaking fees/honoraria from Edwards Lifesciences, Micardia, Valtech, Medtronic and Nycomed; and receiving royalty income from Edwards Lifesciences.
Comments