FDA Approves Baroreflex Activation Therapy Device for Advanced Heart Failure
The Barostim Neo system improved quality of life and exercise capacity in the pivotal BeAT-HF trial.
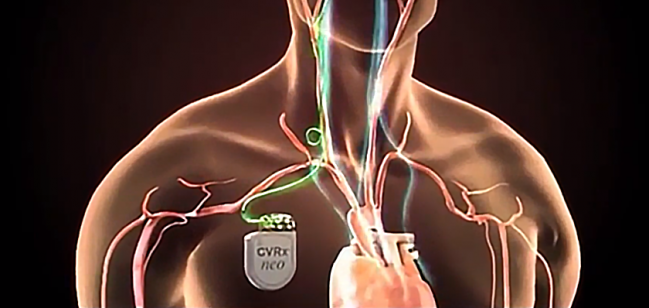
Late last week, the US Food and Drug Administration granted premarket approval to the Barostim Neo baroreflex activation therapy device (CVRx), which is used to improve symptoms in patients with heart failure with reduced ejection fraction who are not eligible for cardiac resynchronization therapy (CRT).
The system, which consists of a subcutaneously implanted pulse generator connected to an electrode placed on the carotid sinus, stimulates the baroreceptors, creating a signal that dampens sympathetic activity and boosts parasympathetic activity.
Acting on the autonomic nervous system in this way was shown to have benefits on top of guideline-directed medical therapy in the BeAT-HF trial. In results reported at the Heart Rhythm Society 2019 Scientific Sessions in May, patients who underwent baroreflex activation therapy had improvements in quality of life and exercise capacity and—in the subset with NT-proBNP levels below 1,600 pg/mL—reductions in levels of that biomarker. In addition, 94% of device-treated patients remained free of major adverse neurological and cardiovascular events through 6 months, exceeding the performance goal of 85%.
A Role for Barostim Neo?
Reaction to those results indicated that the Barostim Neo system is likely to find a role for some patients now that it has been approved.
“We do still see persistent heart failure above and beyond medical therapy. We’re still transplanting patients. We’re still putting left ventricular assist devices in patients,” Shaline Rao, MD (NYU Langone Health, New York, NY), told TCTMD in May. “So I think what we’re looking at is: how do you move the margin on those people who don’t get better despite medical therapy? And are there ways for us to be targeting neurohormonal cascades in ways that we aren’t already?”
Thus, if Barostim Neo can keep patients out of the hospital, it will have clinical value, Rao said. “I do think there’s a role. It’s a smaller subset of heart failure where this is going to be appropriate, but given those are also high healthcare utilizers [with a] high cost to the system, this would be a potentially cost-effective way of managing these patients.”
Clyde Yancy, MD (Northwestern Medicine, Chicago, IL), said at the time that “the data look promising,” although he was cautious when it came to potential clinical impacts, citing remaining questions about the durability of the treatment effects, complications, potential off-target effects, the impact on morbidity and mortality over the longer term, cost-effectiveness, and how patients would feel about this type of intervention.
The Barostim Neo system had received the FDA’s “Breakthrough Device” designation, given to devices that are meant to treat “life-threatening or irreversibly debilitating diseases or conditions” and fill an unmet clinical need. It is indicated for the improvement of heart failure symptoms in patients who remain symptomatic despite the use of guideline-directed medical therapy, are in NYHA class II or III, have an LVEF of 35% or lower and an NT-proBNP level below 1,600 pg/mL, and are not candidates for CRT.
In its announcement, the FDA listed potential complications associated with implantation or use of the system, including “infection; need for reoperation; low blood pressure that may cause dizziness, fainting, and/or falls; nerve damage; surgical or anesthetic complications; allergic reaction; arterial damage; exacerbation of heart failure; stroke; and death.”
As a condition of approval, CVRx must perform a postapproval study to assess the system’s impact on survival and the need for hospitalization.
Barostim Neo previously received CE Mark approval in Europe in September 2014.
Photo Credit: Adapted from barostimtherapy.com
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
US Food and Drug Administration. FDA approves new device to improve symptoms in patients with advanced heart failure. Published on: August 16, 2019. Accessed on: August 19, 2019.


Comments