FDA Approves New Covered Stent Option for Coronary Perforations
The PK Papyrus system is the first approved for this indication in the United States in 17 years.
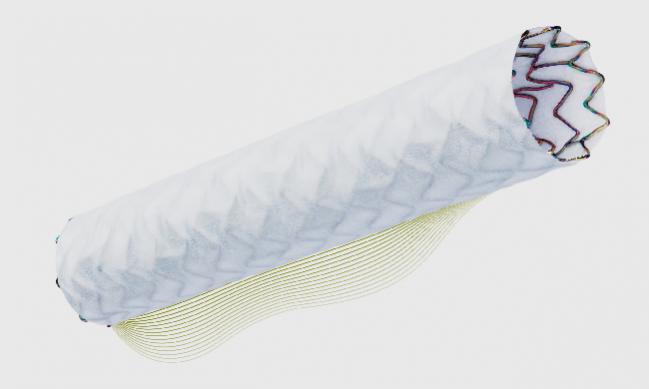
The US Food and Drug Administration (FDA) has approved the PK Papyrus covered coronary stent system (Biotronik) for the treatment of acute coronary artery perforations occurring during PCI.
This is the first US approval of a device for this indication in 17 years, according to an FDA news release.
The agency cleared the balloon-expandable stent and delivery system through its humanitarian device exemption process, which is reserved for devices meant to address a disease or condition affecting no more than 8,000 people in the United States each year.
A review of real-world data on 80 patients showed that the device was successfully delivered in all but four cases; the stent sealed the perforation in 73 of 76 patients. There were two deaths during PCI and then six additional postprocedural deaths in the hospital, five of which were in patients in whom the stent had successfully sealed the perforation.
According to the FDA, the device is contraindicated in patients who cannot undergo standard PCI procedures, including those who are unable to take antiplatelet or anticoagulation therapy, who are allergic to contrast media, who have uncorrected bleeding disorders, or who have a known allergy or hypersensitivity to any of the materials used in the system.
The PK Papyrus system previously received CE Mark approval in Europe in November 2013.
Photo Credit: Biotronik
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
US Food and Drug Administration. FDA approves device for treatment of acute coronary artery perforations. Published on: September 14, 2018. Accessed on: September 14, 2018.


Comments