FDA Gives Thumbs Up to Side Branch Stent for Bifurcation Lesions
The Tryton stent—for use in large side branches—is the first dedicated device for treating bifurcation lesions approved in the United States.
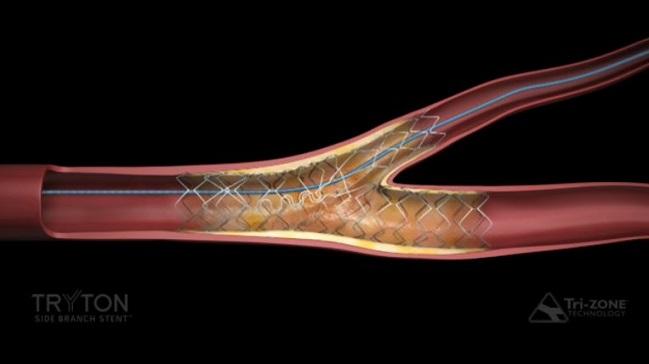
Tryton Medical announced today that its dedicated side branch stent for treating significant coronary bifurcation lesions has been approved by the US Food and Drug Administration (FDA), making it the first such device available in the United States.
The stent is intended for use in bifurcations involving a side branch with diameter stenosis of at least 50%, lesion length no greater than 5.0 mm, and reference vessel diameter of 2.5 to 3.5 mm as well as a main branch with a diameter of 2.5 to 4.0 mm. Any commercially available DES is used in the main branch.
The approval is based on studies showing that the dedicated device is noninferior to provisional stenting—which is standard of care—in patients with large side branches.
TRYTON, the investigational device exemption (IDE) trial, failed to demonstrate noninferiority in terms of target vessel failure in the overall patient population because of a higher rate of periprocedural MI with the side branch stent, but only 41% of patients met entry criteria requiring side branch diameters of 2.25 mm or more by quantitative coronary angiography. In a post hoc analysis restricted to those with large side branches, the dedicated device was shown to be noninferior to provisional stenting; it also reduced percent diameter stenosis in the side branch at 9 months and the need for bailout stenting.
Investigators then initiated a confirmatory study designed in collaboration with the FDA. In patients with large side branches (all but one patient met entry criteria from the IDE trial), the side branch stent was noninferior to provisional stenting in terms of periprocedural MI when compared with a performance goal based on the control arm of the prior trial.
Back when the results of the confirmatory study were released, Philippe Généreux, MD (Morristown Medical Center, NJ), told TCTMD that the Tryton stent facilitates easy access to the side branch, allowing for additional stents as needed, and that it reduces the fear of losing the side branch during a procedure.
“We need a reliable bifurcation technique, and I think Tryton offers one,” he said. “We are doing more and more patients that are turned down for surgery and we are doing more and more complex patients that prefer PCI to CABG, so I think it’s reasonable to see the Tryton stent as a good tool to enhance outcomes in this very complex patient [population].”
Also commenting at the time, however, Issam Moussa, MD (Robert Wood Johnson University Hospital, New Brunswick, NJ), was unsure of what impact the Tryton side branch stent might have on clinical practice, pointing out that it has primarily been studied in lower-risk lesions, which are managed well with the provisional approach.
“I’m uncertain whether if it’s approved it will make headway in the marketplace without more dedicated data in complex bifurcations,” he said last July, noting that it will be more expensive than existing technology.
Tryton Medical has a distribution agreement with Cardinal Health, whose interventional vascular business, Cordis, will serve as the exclusive distributor of the side branch stent in the United States.
Photo Credit: Tryton Medical
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Tryton Medical. Tryton Medical receives FDA approval for Tryton side branch stent to treat significant coronary bifurcation lesions. newsroom.trytonmedical.com/press-release/bifurcation/tryton-medical-receives-fda-approval-tryton-side-branch-stent-treat-signif. Published on: March 6, 2017. Accessed on: March 6, 2017.


Comments