Amputations Still Avoided With Flow-Diverting Therapy for CLTI at 2 Years: PROMISE II
The highly comorbid “no-option” patients have seen sustained pain reduction and avoided amputation.
LAS VEGAS, NV—The diversion of blood flow through arterialization of the deep veins in the foot holds up well at 2 years as a therapy for “no-option” patients with chronic limb-threatening ischemia (CLTI), resulting in sustained limb salvage, data from the PROMISE II study show. The benefit is seen even in very sick patients on dialysis.
The LimFlow (LimFlow SA) device used in the study leverages healthier veins in the leg as conduits to deliver oxygenated blood to the distal venous tissue. In his presentation here at VIVA 2024, Daniel Clair, MD (Vanderbilt University Medical Center, Nashville, TN), said many of the patients had significant tissue loss when they enrolled and were not candidates for surgical bypass or standard endovascular therapies due to the severity of their CLTI.
At 2 years, the limb salvage rate was 65%, with “still very good wound healing out to 2 years in this group of patients, and very low pain scores ultimately after the 3- to 6-month time point in these patients as well,” Clair added.
Immediately after the procedure, pain scores tend to increase in patients due to swelling and inflammation, but once that subsided the mean pain score seen in the trial was 1.2 on a scale of 0 to 10, with 69% of patients reporting being pain free at 2 years. This is consistent with the 1-year data, which showed the mean pain score to be 1.4.
During the discussion following the presentation, Clair said 35%-40% of patients in the trial needed additional interventions in the first year, most commonly transmetatarsal amputation.
Speaking with TCTMD, moderator Andrew Holden, MBChB (Auckland Hospital, New Zealand), said the reduction in pain is impressive for these highly comorbid patients.
“I don't think we really understand the mechanism. We see some kind of reduction in peripheral vascular resistance from the bed, and that's associated with a reduction in pain,” he said. “I would say it's observational without a mechanism.”
The US Food and Drug Administration approved the LimFlow device in 2023 on the basis of 6-month data from PROMISE II, making it the only device of its kind for transcatheter arterialization of deep veins.
Holden said while the potential for significant reduction in pain is important to patients, there are caveats that need to be conveyed prior this type of procedure.
“You really need to be honest, because most of these patients will end up needing an ancillary procedure like a transmetatarsal amputation,” he noted. “So, they're dealing with quite a lot of things. They are having to manage mobilizing on a partial amputation and they're going to have a tough time for a month or two after this procedure, but then they'll get a very good functional result that is quite beneficial.”
High Rates of Wound Healing Seen
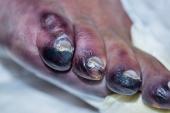
PROMISE II was a single-arm study that screened 219 no-option CLTI patients from 20 centers in the United States, enrolling 105 individuals (median age 70 years; 31% female) between 2018 and 2022. More than three-quarters had diabetes, 91% had hypertension, nearly 40% had chronic kidney disease, 20% had end-stage renal disease, and 74% had undergone a prior target-limb revascularization. All had a nonhealing foot ulcer or gangrene and were Rutherford class 5 or 6. Patients were classified as “no-option” due to lack of runoff target for traditional intervention (97%) and lack of suitable conduit for bypass (3%). Arteriovenous crossing locations were posterior tibial artery in 76%, peroneal artery in 19%, and tibioperoneal trunk in 6%.
At 2 years, 65.8% of patients were in Rutherford class 4 or below, with 54.3% in Rutherford class 0, Clair said. In 82% of patients, wounds were completely healed or healing, with a mean wound area of 0.1 cm2.
Taken together with the prior results from the PROMISE I early feasibility trial, Clair said the data suggest no differences in rates of limb salvage by age, sex, race, baseline Rutherford classification, diabetes, or dialysis. The pooled survival rate of both trials at 2 years is 72%, limb salvage is 68%, and amputation-free survival is 49%.
Clair also showed another analysis that compared the pooled PROMISE cohorts with no-option CLTI patients who underwent alternative strategies. That analysis showed that the amputation-free survival with the flow-diverting therapy was 58% at 1 year versus just 34% for the alternatives.
Clair stressed in his presentation that the therapy is for the sickest CLTI patients, noting that due to the risk of transmetatarsal amputation, he would not recommend the procedure for those with rest pain. He also noted that other interventions that have been required in these patients include managing blood flow if it is either too little or too much on duplex ultrasound.
I don't think we really understand the mechanism. We see some kind of reduction in peripheral vascular resistance from the bed, and that's associated with a reduction in pain. Andrew Holden
Panelist Matthew Menard, MD (Harvard Medical School, Boston, MA), asked Clair to clarify the importance of vessel patency to long-term outcomes.
Clair noted that despite the fact that many of the revascularizations will fail within a year, “there is clearly a distinct new arterial blood distribution that develops in the foot.” He added: “The first several patients I treated all failed within a year and all retained their limbs for the entire time they were alive.”
As with Holden, Clair said he doesn’t fully understand how or why that happens.
“I think we have yet to discern the mechanism by which that occurs, but there clearly is some angiogenesis, possibly some recruitment through alternative pathways to get blood flow into arterioles that were not patent or were hibernating before,” he said.
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Clair D. Two-year outcomes from the PROMISE II trial of transcatheter arterialization of the deep veins. Presented at: VIVA 2024. November 5, 2024. Las Vegas, NV.
Disclosures
- The trial was sponsored by LimFlow.
- Clair reports honoraria from Boston Scientific and Endologix as well as consulting for Boston Scientific, Caeli Vascular, ElastiMed, Endologix, LimFlow, Medtronic, Nectero, and Vesteck.




Comments