Intra-arterial Lytics Don’t Help After Stroke Thrombectomy, but Questions Persist
Two new trials saw no impact of thrombolytics in reducing disability at 90 days, but some positive signals offer hope.

For patients with acute ischemic stroke due to a large-vessel occlusion (LVO), administering intra-arterial thrombolytics after successful endovascular thrombectomy does not significantly improve outcomes, two randomized trials indicate.
Adding intra-arterial urokinase or tenecteplase didn’t significantly increase the likelihood of surviving without disability at 90 days in the POST-UK and POST-TNK trials, respectively, although the proportion of patients to meet that benchmark was numerically higher in both studies.
Both studies were published online this week in JAMA.
The findings come nearly 3 years after publication of the CHOICE trial, a phase IIb study demonstrating a large and significant benefit of adjunctive intra-arterial alteplase after successful stroke thrombectomy.
Asked about the discrepancy between that trial and the latest ones, Pooja Khatri, MD (University of Cincinnati, OH), who was not involved in any of the studies, pointed out that CHOICE was a much smaller study that ended prematurely and included patients with a wider range of reperfusion results after thrombectomy. In addition, most patients in CHOICE received IV thrombolytics before the procedure, compared with none in POST-UK and POST-TNK.
In that context, these newer data “are promising in terms of a therapeutic strategy with thrombolysis to attack distal visualized clots and maybe impaired microcirculatory reperfusion, or the no-reflow phenomenon as we like to call it,” she said, adding, however, that “I’m disappointed that the potential effect isn’t bigger.”
Looking to Improve Thrombectomy Results
Over the past decade, multiple randomized trials have established endovascular thrombectomy as the go-to treatment for patients with acute ischemic strokes caused by LVOs. Still, fewer than half of treated patients are disability free by 90 days, possibly stemming from residual problems in the microvascular circulation.
Though CHOICE indicated that adjunctive intra-arterial thrombolytic therapy may help address this issue and improve patient outcomes, the study’s limitations complicated interpretation.
POST-UK and POST-TNK, using two different thrombolytics, provide more insights into this question. Both trials enrolled patients presenting with acute ischemic stroke caused by an LVO within 24 hours of when they were last known to be well who underwent stroke thrombectomy and achieved a successful result (eTICI 2c-3 perfusion); those who received IV thrombolytics before the procedure were excluded. Patients were then randomized to receive intra-arterial thrombolytic therapy (with urokinase in POST-UK and tenecteplase in POST-TNK) or no intra-arterial therapy. The primary efficacy endpoint in both trials was freedom from disability at 90 days, defined as a score of 0 or 1 on the modified Rankin Scale, 0 being no symptoms and 6 being death.
POST-UK, reported by lead author Chang Liu, MD (Second Affiliated Hospital of Chongqing Medical University, China), and colleagues, was conducted at 35 comprehensive stroke centers in China with 535 participants (median age 69 years; 41.8% women). POST-TNK, reported by Jiacheng Huang, MD (Second Affiliated Hospital of Chongqing Medical University), was conducted at 34 comprehensive stroke centers in China with 540 patients (median age 69 years; 40.9% women).
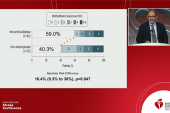
The primary efficacy outcome was numerically, but not significantly, higher with the addition of intra-arterial urokinase in POST-UK (45.1% vs 40.2%; adjusted risk ratio 1.13; 95% CI 0.94-1.36) and tenecteplase in POST-TNK (49.1% vs 44.1%; adjusted risk ratio 1.15; 95% CI 0.97-1.36), with consistent results across subgroups.
The authors of both papers note that the confidence intervals don’t exclude a clinically meaningful benefit of adjunctive intra-arterial thrombolysis.
In terms of safety, there were no significant differences in 90-day mortality or in the incidence of symptomatic intracranial hemorrhage within 48 hours between arms in either trial. In POST-TNK, however, intra-arterial tenecteplase was associated with trends toward less mortality (16.0% vs 19.3%; P = 0.16) and more hemorrhage (6.3% vs 4.4%; P = 0.35).
Lingering Questions
One lasting uncertainty since the CHOICE results came out is how much of the effect was due to the thrombolytics acting on remaining visualized clot in the macrocirculation versus thromboses in the microcirculation, Khatri said, noting that in both POST-UK and POST-TNK, intra-arterial treatment seemed to have more benefit in patients with an angiographic eTICI score of 2c than in those with complete reperfusion (eTICI 3).
“It leaves me with this question of whether we’re on the right track for attacking the no-reflow phenomenon,” Khatri said. “It’s all been kind of theoretical that it’s reversible microthromboses. We know there are other biological processes in play in that microcirculatory space, but it certainly energizes the question further.”
Khatri noted that, currently, “it’s not uncommon in clinical practice to squirt a little [intra-arterial] thrombolysis at residual clot that remains in the distal vessels after we take the proximal vessel occlusion out.”
But there are still trials ongoing, and at this point, the accumulated evidence is not sufficient to recommend this approach more broadly, she said. “At the end of the day, the primary outcomes were negative [in POST-UK and POST-TNK]. And so we need to see more definitive data. I don’t see us having a strong recommendation. I could see a weak recommendation or a mixed recommendation [in future guidelines].”
In an accompanying editorial, Diederik Dippel, MD, PhD (Erasmus University Medical Center, Rotterdam, the Netherlands), and colleagues say the evidence is not strong enough to support implementing intra-arterial thrombolysis into clinical practice, noting that additional trials like TECNO and 2BE3 will provide more data.
“Nevertheless, the POST-UK and POST-TNK investigators should be commended for their effort of conducting two trials,” Dippel et al write. “Their results, although nonsignificant, do not exclude a relevant treatment effect. The current ongoing trials may provide more evidence to the efficacy of periprocedural intra-arterial thrombolytics in ischemic stroke patients.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Liu C, Guo C, Li F, et al. Intra-arterial urokinase after endovascular reperfusion for acute ischemic stroke: the POST-UK randomized clinical trial. JAMA. 2025;Epub ahead of print.
Huang J, Yang J, Liu C, et al. Intra-arterial tenecteplase following endovascular reperfusion for large vessel occlusion acute ischemic stroke: the POST-TNK randomized clinical trial. JAMA. 2025;Epub ahead of print.
Dippel DWJ, Khan CF, Schoon BA. Intra-arterial thrombolytics during thrombectomy for ischemic stroke—end of the story or a new beginning? JAMA. 2025;Epub ahead of print.
Disclosures
- POST-UK was supported by the National Natural Science Foundation of China, Natural Science Foundation of Chongqing, Chongqing Technology Innovation and Application Development Project, the National Natural Science Foundation of China Major Program, and China Postdoctoral Science Foundation. The study drug was provided by Wuhan Humanwell Pharmaceutical Co.
- POST-TNK was supported by the National Natural Science Foundation of China, Natural Science Foundation of Chongqing, Chongqing Technology Innovation and Application Development Project, and China Postdoctoral Science Foundation. The study drug was provided by China Shijiazhuang Pharmaceutical Company Recomgen Pharmaceutical.
- Dippel, Huang, and Liu report no relevant conflicts of interest.




Comments