It’s Been 1 Year Since Medicare’s NCD for Carotid Stenting: What Now?
In high- and standard-risk patients CAS and TCAR look good, but some worry about skill sets, learning curves, and territorialism.
LAS VEGAS, NV—One year after Medicare’s National Coverage Determination (NCD) expanded access in the United States to carotid artery stenting (CAS), data reassure on the safety and efficacy of the procedure in both high and standard-risk patients, but not everyone is convinced that widening the pool of who’s eligible is necessarily a good idea.
In two presentations last week at VIVA 2024—PERFORMANCE II and the ROADSTER 3 postapproval study—a transcarotid artery revascularization (TCAR) procedure and a three-in-one CAS system with integrated embolic protection showed good short- and long-term outcomes, with low rates of stroke.
In a separate debate about the new NCD, however, experts clashed over whether current evidence is adequate enough to consider the procedure equal to or better than surgery, and whether proper protections are in place to safeguard patients from operators who don’t have the necessary skills or experience to embark on CAS cases.
“If you look at the actual data, in the last 365 days since the change in NCD, there's not really been a great [surge] of people doing carotid stents,” noted Sean P. Lyden, MD (Cleveland Clinic, OH), who moderated the debate.
The NCD came about following a formal request to the Centers for Medicare & Medicaid Services (CMS) from the Multi-Specialty Carotid Alliance (MSCA)—which includes physicians specializing in neurology, neuroradiology, neurosurgery, interventional radiology, vascular surgery, vascular medicine, and interventional cardiology. Under the NCD, CMS said carotid stenting is reasonable and necessary in Medicare patients with symptomatic carotid artery stenosis ≥ 50% and those with asymptomatic carotid artery stenosis ≥ 70%.
Christopher J. White, MD (Ochsner Medical Center, New Orleans, LA), a MSCA member, spoke in favor of the NCD to improve care in the debate session.
“It supports the right patient getting the right treatment at the right time,” he said. “There's nothing that makes healthcare more effective and better for patients than meeting those criteria. This is patient-centered decision-making.”
William A. Gray, MD (Main Line Health/Lankenau Heart Institute, Wynnewood, PA), a panelist at the debate who presented 2-year outcomes of the PERFORMANCE II trial in a late-breaking data session, told TCTMD that he hopes the NCD will help the US catch up with the rest of the world in terms of offering CAS as a treatment option when appropriate.
“What it does is it opens the door for us to have a conversation with our patients about their specific needs and all three options—TCAR, carotid endarterectomy [CEA], and carotid stenting—and what might be best suited to them,” he said. “I think the next few years we're going to try to figure out which patients are best suited to which procedure, but the vast majority of them fall into kind of a Venn diagram of overlap [where] any of the three procedures work well for those at low risk.”
What it does is it opens the door for us to have a conversation with our patients about their specific needs and all three options—TCAR, carotid endarterectomy [CEA], and carotid stenting. William A. Gray
But Margaret C. Tracci, MD, JD (UVA Health, Charlottesville, VA), noted in the debate that the existence of conflicting society requirements for operators performing CAS “lays open the opportunity not for excellent operators with great standards, but for territorial pushing and shoving, or just dereliction of duty on this front.”
Tracci is chair of the advocacy council for the Society for Vascular Surgery, which opposed the new NCD. Among the groups’ primary reasons they cited following the CMS decision were quality of care and patient safety concerns, as well as a belief that the move was premature given the pending results of the CREST 2 trial, which is comparing intensive medical therapy alone or in combination with CAS or CEA.
In the debate, Tracci argued that the evidence of equipoise between CAS and CEA is chiefly composed of “expert operators in very controlled settings” and said strict enrollment criteria may not reflect real-world operators, patients, or outcomes. Tracci added that she worries about the NCD opening up “the potential for some pretty bad outcomes as people climb the [CAS] learning curve.”
Looking at New Data
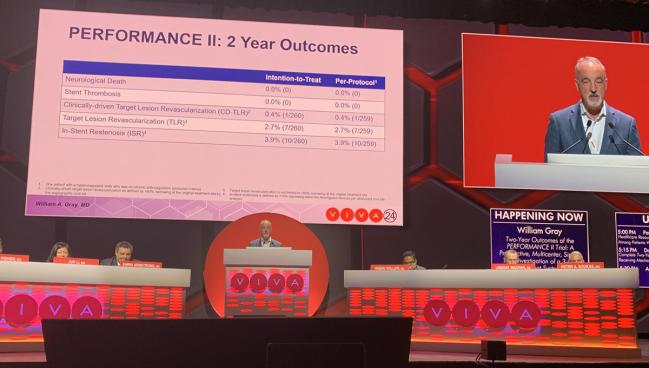
Gray’s PERFORMANCE II data, which involved 305 mostly asymptomatic high-surgical-risk patients treated with the Neuroguard IEP three-in-one carotid stent system (Contego Medical) with integrated embolic protection, showed a 30-day stroke rate of 1% in a per-protocol analysis and 1.6% in an intention-to-treat analysis. The rate of death/stroke was 1.6% and the rate of death/stroke/MI was 2.3%. The rate of 30-day all-stroke plus ipsilateral stroke was 1.8% through 1 year. All strokes were minor, with no cases of major stroke, stent thrombosis, or neurologic death through 2 years. The majority of patients in the trial were considered high risk based on physiologic criteria, with 25% having high-risk anatomic criteria, and 28% having both high-risk physiologic and anatomic criteria.
In-stent restenosis occurred in 3.65% at 1 year and 3.9% at 2 years. Clinically driven TLR was 0.4% at 2 years, while non-clinically driven TLR rate was 2.7%.
A second presentation of new CAS data involved the ROADSTER 3 postapproval study evaluating the safety and efficacy of TCAR with the Enroute stent system used in conjunction with the ENROUTE neuroprotection system (both Boston Scientific) in 344 patients at standard surgical risk.
There was a 0.9% incidence of stroke/death/MI at 30 days in the intent-to-treat population. No deaths or MIs were reported through 30-day follow-up. The rate of cranial nerve injury at 30 days was 0.6%.
Meghan Dermody, MD (Penn Medicine, Lancaster General, PA), who presented the ROADSTER 3 results, said there is a paucity of data to support TCAR in the standard-risk population, making studies like this one important in filling gaps for clinicians who have patients sitting in front of them who they need to evaluate for best management options.
She said the ROADSTER 3 investigators have begun to enroll patients in a 5-year follow-up arm looking at stent patency and neurologic outcomes over time for further evaluation of the durability of the procedure in this patient population.
An ‘Open Playground’ in Need of Direction
In response to a question about how she expects TCAR to shake out in standard-risk patients, Dermody said the main message is careful evaluation and individualization of therapy.
“I think what we have now is an open playground and we really need some direction on what to do with specific lesions in specific patient populations,” she added.
According to Tracci, what’s needed now in addition to better understanding of patient selection is a collaborative process to tackle the learning and training curve for CAS operators as well as continued careful collection of real-world outcomes data.
I think what we have now is an open playground and we really need some direction on what to do with specific lesions in specific patient populations. Meghan Dermody
Responding to Tracci’s argument that a limited number of operators have the required skill set to do CAS, Gray said that’s not specific to this issue, noting that regardless of the type of procedures a hospital offers, it has a duty to ensure proper oversight and a robust credentialling process for its operators and teams.
“To pull out carotid stenting as a problem child, I think is disingenuous, to be honest with you,” he said.
D. Christopher Metzger, MD (OhioHealth Riverside Methodist Hospital, Columbus), a panelist during the debate, said the 1% rates of stroke and other positive data being seen in CAS trials suggest that in the right hands, there is equivalence between stenting and CEA, but that the management decisions will continue to require objective clinical decision-making.
“I'd be the first to call the surgeon myself if [the patient would do] better with endarterectomy than a carotid stent. Just because I don't do carotid endarterectomy doesn't mean you can't participate in the shared decision-making,” he added.
Gray said the data will continue to evolve over the next few years, and “if CREST 2 turns out to be a win for medical therapy, then stenting and surgery are going to decline and that’s fine.”
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Gray WA. Two-year outcomes of the PERFORMANCE II trial: a prospective, multicenter, single-arm investigation of a 3-in-1 carotid stent system with integrated embolic protection. Presented at: VIVA 2024. November 4, 2024. Las Vegas, NV.
Dermody M. Prospective, Multicenter evaluation of transcarotid artery revascularization in standard-risk patients: 30-day outcomes of the ROADSTER 3 Study. Presented at: VIVA 2024. November 4, 2024. Las Vegas, NV.
Disclosures
- Gray reports grant/research support and consultant fees/honoraria from Contego Medical.
- Dermody reports proctoring for Silk Road Medical.




Comments