Low 1-Year Stroke Risk After Commercial Watchman Use
The registry findings support the effectiveness of transcatheter LAA occlusion as practiced in the US, a researcher says.
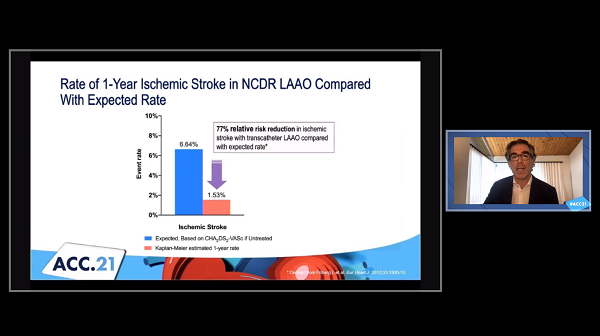
The estimated 1-year rate of ischemic stroke after implantation of the Watchman left atrial appendage (LAA) closure device (Boston Scientific) was 1.53% in its first few years of commercial use in the United States, which encompassed a relatively high-risk population of patients with atrial fibrillation (AF), registry data show.
That’s substantially lower than would be expected in a cohort of patients with a similar thromboembolic risk based on CHA2DS2-VASc score who are not taking oral anticoagulation (6.64%), Matthew Price, MD (Scripps Clinic, La Jolla, CA), reported Saturday during the virtual American College of Cardiology (ACC) 2021 Scientific Session.
The low thromboembolic event rate observed in the study, which used information from the ACC’s National Cardiovascular Data Registry (NCDR) LAAO Registry, “supports the early-to-midterm clinical effectiveness of transcatheter LAAO as it is being currently utilized in the United States,” Price said.
“The not-insubstantial risks of bleeding in the early postdischarge period and of the risk of death unrelated to thromboembolism should be incorporated into a patient-centered [decision] when selecting the appropriate stroke prevention strategy for A-fib patients unable to take long-term oral anticoagulation,” he added.
Commenting for TCTMD, Christine Albert, MD (Smidt Heart Institute at Cedars-Sinai, Los Angeles, CA), said the study was limited by its lack of a contemporary comparator group, pointing out that the ischemic stroke rate estimated using historical data was “rather high.” Even so, she said, “I think overall it’s very reassuring because you would at least expect that [the ischemic stroke rate] would be higher than what they found” given the level of risk in the registry patients.
Higher-Risk Patients in the Real World
Price noted that the populations treated in the pivotal Watchman trials—PROTECT-AF and PREVAIL—differed from the types of patients that have been treated following US Food and Drug Administration approval of the device, driven by requirements for coverage laid out by the Centers for Medicare & Medicaid Services (CMS). In particular, patients in the trials had to be eligible for long-term warfarin therapy, whereas CMS has said patients should be suitable for short-term warfarin therapy but unable to take long-term oral anticoagulation.
Not surprisingly, Price said, a previous report from the LAAO Registry showed that patients treated after Watchman’s approval were higher risk than the trial participants—they tended to be older, had higher rates of prior stroke/TIA and diabetes, and had higher estimated risks of thromboembolic events and bleeding. Nevertheless, the registry patients had lower rates of in-hospital complications than were seen in the trials.
For this new study, Price and colleagues examined 1-year outcomes in the LAAO Registry. The analysis included 36,681 patients (mean age 76 years; 59% men) who underwent an attempted or successful Watchman procedure between 2016 and 2018. Mean CHA2DS2-VASc score was 4.8, mean HAS-BLED score was 3.0, and more than two-thirds of patients (69.5%) had experienced prior clinically relevant major bleeding. Median follow-up was 373 days.
The primary endpoint was ischemic stroke, with 1-year rates calculated using the Kaplan-Meier method to account for variable lengths of follow-up. Despite the high-risk nature of the patients included, the estimated stroke rate (1.53%) was consistent with prior RCTs and registries of the Watchman device, Price reported.
The estimated 1-year rate of major bleeding was 6.2%, with most bleeds occurring between discharge and 45 days (the in-hospital rate was 1.3%), he noted. Although postprocedural pharmacotherapy was not available for this study, Price said, a prior analysis showed that 80% to 90% of patients in this registry were discharged on oral anticoagulation.
“In context, the relatively low ischemic stroke [rate] cannot be ascribed to LAAO alone because patients do take a short term of postprocedural pharmacotherapy, but certainly given the risk of the population, the results are reassuring that how we’re practicing medicine now in the United States—putting in a Watchman and using these pharmacotherapies afterwards—translates into a low thromboembolic event rate,” he said during a panel discussion after his presentation.
All-cause mortality occurred at an estimated rate of 8.52% during the first year, with most of the deaths (60.3%) attributed to noncardiovascular causes; one-third (32.6%) were classified at CV deaths.
Reliance on Shared Decision-making
Albert noted that the estimated bleeding and mortality rates were high, but said it “speaks to the patient population that’s getting these devices at this point. They’re very sick in a lot of ways.”
Whether those rates are higher than would be seen in a similar population of patients who cannot take long-term anticoagulation and are not treated with the Watchman is hard to say without a randomized trial, but such a trial is unlikely to happen at this point because the device is already being used clinically, Albert said. PROTECT-AF and PREVAIL suggest that Watchman is at least comparable to oral anticoagulation, she added.
“I think we have enough data to know that the concept of occluding the left atrial appendage probably does prevent stroke,” Albert said, noting that there could be some debate about whether withholding LAA occlusion in patients who can’t receive oral anticoagulation when there might a benefit would be ethical at this point. Ongoing trials comparing the Watchman device with the direct oral anticoagulants in patients who are eligible for long-term anticoagulation will provide some insights into the effectiveness of percutaneous LAA occlusion, she said.
Though the trial didn’t involve transcatheter LAA occlusion, LAAOS III—presented earlier in the day today—provided impressive results indicating a reduction in ischemic stroke with surgical LAA occlusion in patients undergoing cardiac surgery for another reason, Albert noted. “So the concept that left atrial appendage occlusion prevents stroke is stronger now because of that randomized trial.”
In a panel discussion after Price’s presentation, Jodie Hurwitz, MD (Medical City Hospital, Dallas, TX), hinted that a stronger guideline recommendation for the Watchman might be warranted.
Price responded by noting that an attempt to do a randomized trial in patients with contraindications to long-term oral anticoagulation—ASAP-TOO—was discontinued due to low enrollment, making it unlikely that there will be randomized data for this cohort. But the findings from the current study are reassuring, he said.
Albert, too, was hesitant to support any changes to guidelines in the absence of a randomized trial. “I think it’s hard to get to a class I indication without that,” she said, also highlighting the data from the LAAO Registry showing a low stroke rate in a relatively high-risk population. “I think that it’s at least reassuring that it wasn’t 4% or something like that. So it’s good data to know that it does appear to be working, but I just don’t know if it will change the guidelines.”
For now, the choice to use the Watchman is “really at ‘shared decision-making’ still,” Albert said. She said she considers using the device when a patient can’t tolerate long-term anticoagulation and she wants to offer some protection against stroke. “But there are patients in this category that choose not to have a Watchman and can’t be anticoagulated. So that’s why the recommendations I think are the way they are, is that you really do have to be allowed to put up what the data are for the patient so that they can make an informed decision.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Price MJ. 1-year clinical outcomes following Watchman transcatheter LAAO for stroke prevention in patients with atrial fibrillation: a report from the NCDR LAAO Registry. Presented at: ACC 2021. May 15, 2021.
Disclosures
- The study was supported by the ACC’s NCDR and the National Heart, Lung, and Blood Institute.
- Albert reports an investigator-initiated grant from St. Jude Medical that is unrelated to LAA occlusion.




Comments