New Laser-Based Imaging Modality in Development for Atherosclerosis Assessment
The technology may one day help guide interventions, identify causes of cryptogenic stroke and breathe new life into the vulnerable plaque hypothesis.
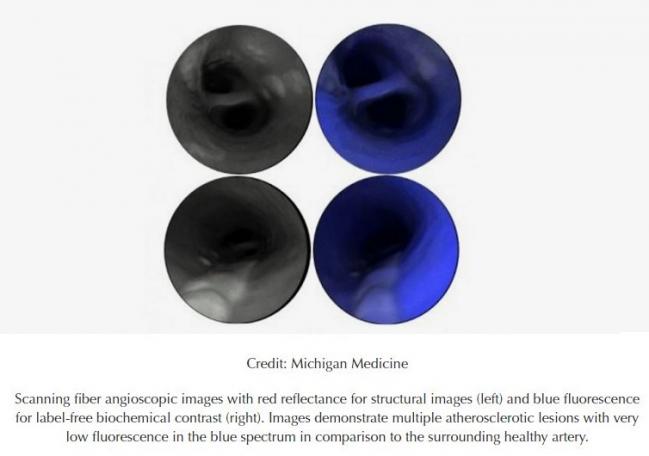
Though still in preclinical evaluation, a new imaging modality may someday improve assessment of atherosclerotic plaques in the carotids and elsewhere, a proof-of-concept study suggests.
Originally developed for the early detection of cancer cells in the GI tract, the scanning fiber endoscope (SFE) consists of a single optical fiber on the end of a highly flexible catheter and blue, green, and red lasers to scan the inner surface of the vessel. Light reflectance and tissue autofluorescence are then reconstructed into high-resolution images providing structural, biochemical, and biological information in real time.
“By synergistically combining high-definition endovascular endoscopy, laser-generated fluorescence, and molecular imaging of key biological processes, this catheter-based technology holds the potential to become a new platform for research, diagnosis, prognosis, and image-guided local therapy in atherosclerosis and cardiovascular disease,” lead author Luis Savastano, MD (University of Michigan, Ann Arbor), and colleagues write in a study published online February 10, 2017, ahead of print in Nature Biomedical Engineering.
Researchers have tested the technology in carotid arteries from human cadavers and surgical specimens, showing that it is possible to classify plaques by their severity, spanning early, intermediate, advanced, and complicated (which includes intraplaque hemorrhage, ulcerated plaque, and intraluminal thrombus) categories.
“We also demonstrated that the superior image resolution of this technology revealed intravascular thrombi and surface thrombogenic lesions, even in cases not detected by conventional diagnostic modalities,” the authors explain. “Then, by targeting proteolytic activity of complicated atherosclerotic plaques with a fluorescence agent activated by matrix metalloproteinases, we were able to identify vulnerable regions of weakened fibrous cap at a resolution far beyond clinically available technology.”
The investigators also showed the feasibility of using SFE in vivo by imaging infrarenal aortoiliac vessels of New Zealand rabbits.
An Unmet Clinical Need
Savastano and colleagues note that recent decades have seen the emergence of various forms of intraluminal imaging of atherosclerosis, including endovascular angioscopy, IVUS, optical coherence tomography (OCT), and optical spectroscopy.
“However, a standalone platform capable of generating real-time structural, chemical, and biological images of large vascular surfaces to reveal high-definition pathological hallmarks of vulnerable and complicated atherosclerotic lesions, both for diagnostic and prognostic purposes and to guide intraluminal interventions, is still an unmet clinical need,” they say.
The SFE, invented and developed by Eric Seibel, PhD, a mechanical engineering research professor at the University of Washington, Seattle, may help fill that need, Savastano told TCTMD.
Positive attributes of the technology include the ability to generate images with a long depth of focus and very high resolution, he said, noting that currently available angioscopes have resolutions up to about 10,000 pixels and this new angioscope has a resolution up to about 250,000 pixels. Investigators can see features on the tissue surface that were impossible to image previously, he added.
The device is also small, he said. Current prototypes have an outer diameter of 1.2 mm, but researchers are working on bringing that down to 0.85 mm, which would allow navigation in very small vessels.
Savastano said an advantage over other technologies that image the lumen of the vessels is that the SFE is a front-view device that provides “down-the-barrel” views in real time, whereas OCT and IVUS, for example, are lateral-view devices that require crossing regions of interest followed by pullbacks.
A Long Way to Go
If the SFE technology is eventually proven in clinical studies—which Savastano acknowledged is a long-term prospect—it could have multiple applications in practice. The patients most likely to benefit, he said, are those with cryptogenic stroke. Using SFE, clinicians could pinpoint the origin of the thrombus that caused the event, which often is suspected to be in nonstenotic complicated plaques at the carotid bifurcations.
The technology might also be useful for identifying patients who have vulnerable plaques that place them at high risk for future cardiovascular events and might be suitable for the initiation or intensification of preventive therapies.
And finally, Savastano said, because it provides a real-time look inside the vessel with high resolution and a wide field of view, the device could be used to guide interventions and monitor for procedural complications.
But much more work needs to be done before SFE can be brought to the clinic. The classification system developed by Savastano et al needs to be validated in clinical settings, for example.
Also, the authors point out, “standalone SFE provides limited information on the vertical structure of tissues. A combination of SFE with cross-sectional imaging technology, such as OCT, will allow further characterization of different types of fibroatheroma and precise quantification of fibrous cap thickness.”
Savastano said that he envisions this technology being used in all vascular fields, but it remains to be tested in coronary and peripheral vessels. Preliminary results in the carotids suggests that use in the coronaries is a possibility, but further developments in the size and flexibility of the device will be needed, he added.
More generally, he said, endovascular techniques need to be optimized before clinical translation.
“There is an unmet clinical need for improved imaging modalities to see complicated and vulnerable plaques, especially in carotid arteries,” Savastano said. “We have proven the concept with this paper that scanning fiber angioscopy is able to generate high-resolution images of the structure and the biochemical makeup of the plaques and biological pathways within the plaques, and therefore has the potential to become a new imaging platform for imaging of atherosclerosis.”
Photo Credit: Michigan Medicine, University of Michigan
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Savastano LE, Zhou Q, Smith A, et al. Multimodal laser-based angioscopy for structural, chemical and biological imaging of atherosclerosis. Nat Biomed Eng. 2017;Epub ahead of print.
Disclosures
- The study was supported by a Cerebrovascular Research Award, Joint Section on Cerebrovascular Surgery of the American Association of Neurological Surgeons and Congress of Neurological Surgeons, and the National Institutes of Health.
- Savastano reports no relevant conflicts of interest.
- Seibel reports participating in royalty sharing with the University of Washington, which has ownership of patents that may gain or lose financially through publication of the study.


Comments