No Mortality Signal for iFR in Real-world Use: SWEDEHEART
DEFINE-FLAIR showed higher 5-year mortality with iFR vs FFR, sparking controversy. These observational data reassure.
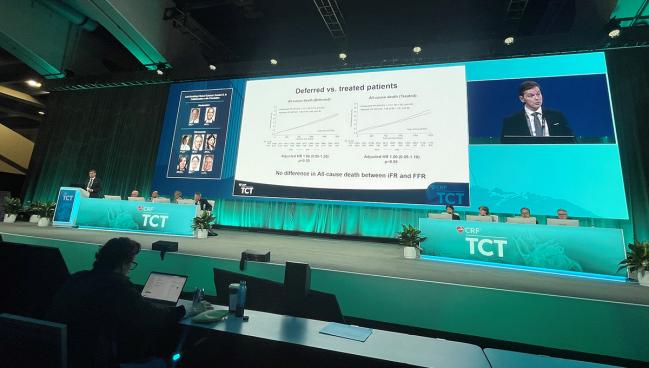
DEFINE-FLAIR and iFR-SWEDEHEART, two trials with similar designs conducted by different research groups, both initially showed that iFR matched FFR for MACE (death, nonfatal MI, and unplanned revascularization) at 1 year. Yet there was a trend toward higher mortality with iFR in DEFINE-FLAIR.
Then, this spring at EuroPCR 2023, 5-year follow-up from DEFINE-FLAIR showed significantly higher mortality with iFR versus FFR (HR 1.56; 95% CI 1.16-2.09), with the signal apparent in treated patients only. No such difference had been seen at 5 years in iFR-SWEDEHEART.
Matthias Götberg, MD, PhD (Skåne University Hospital, Lund, Sweden), who presented the new registry results Wednesday at TCT 2023, said that going into their analysis he had not been too concerned that the signal would turn out to represent real harm. Rather, “we thought it was a little bit puzzling” to see the increased mortality only in the patients who actually had revascularization, not those who waited, he explained. “If you had an index that didn’t perform well, you’d think it would be among the deferred patients.”
Thus, the researchers decided to dig deeper by using the SWEDEHEART national registry, known for its comprehensive follow-up and large size, Götberg said. “That gives us really the power to say with a great deal of certainty whether there was any difference or not.”
Answering this question is “important because there’s obviously been a lot of controversy in the past about iFR, FFR, and which is better,” he commented.
There’s obviously been a lot of controversy in the past about iFR, FFR, and which is better. Matthias Götberg
At a media briefing ahead of his TCT presentation, Götberg concluded that “overall we don’t really see any differences when we have a large enough sample size.” The DEFINE-FLAIR mortality disadvantage for iFR seems to be “a play of chance,” he said.
Ajay Kirtane, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY), who moderated a press conference at which the results were presented, agreed that “there was definitely a fair amount of controversy” generated by the 5-year DEFINE-FLAIR data during the EuroPCR meeting and thereafter, to the point that some were calling for a rethink of the guidelines. The large amount of data contained in the SWEDEHEART registry presents a good way to examine the signal, he added.
No Interaction With Treatment
Götberg and colleagues looked at 5-year follow-up data for 42,887 patients in the SWEDEHEART national registry who’d undergone iFR or FFR. In this real-world population, the risk of MACE was similar between the two testing modalities (adjusted HR 0.99; 95% CI 0.93-1.05), as was the risk of death (adjusted HR 1.04; 95% CI 0.94-1.14). There was no difference in either outcome based on whether patients were treated or deferred. MI and urgent revascularization risks also were similar between iFR and FFR.
“The observed difference in mortality between iFR and FFR among treated patients in DEFINE-FLAIR could not be verified in this large, nationwide, real-world, all-comers registry analysis, Götberg concluded.
Although many people have invested time and energy in FFR, which has its proponents, Götberg pointed out that iFR is faster to do, avoids the side effects associated with adenosine inherent to FFR, and is cost-effective. The savings come from no adenosine cost and the fact that more procedures tend to be deferred with iFR versus FFR, he noted to TCTMD.
Kirtane, speaking with the media, cited similar advantages to iFR. Given that revascularization is deferred more often with that option, he added, it was especially reassuring to see the lack of mortality difference among the untreated patients.
It just educates us about the fact that we have to look at everything from all sides and take our time and consider what it is we actually [see]. Margaret B. McEntegart
B. Hadley Wilson, MD (Sanger Heart & Vascular Institute, Atrium Health, Charlotte, NC), president of the American College of Cardiology and a panelist in the press conference, asked if there are any remaining subgroups where FFR comes out ahead: “Do you see any role for FFR after all of this?”
None leapt out when they did these analyses in SWEDEHEART, Götberg said. Sex, age, smoking status, comorbidities, disease history, lesion location, and the year of enrollment all appeared to have no impact.
Margaret B. McEntegart, MD, PhD (NewYork-Presbyterian/Columbia University Irving Medical Center), in the late-breaking trial session where Götberg gave his presentation, made the case for looking not just at randomized trials but also at observational studies.
“I think what this situation illustrates is the complementary value of the two different types of data. Sometimes we get incredibly focused on one type of data versus the other. Sometimes observational data misleads us: we’ve seen that before as well,” commented McEntegart. “So I think it just educates us about the fact that we have to look at everything from all sides and take our time and consider what it is we actually [see].”
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Götberg M. Long-term clinical outcomes after iFR vs. FFR-guided coronary revascularization – insights from the SWEDEHEART national registry. Presented at: TCT 2023. October 25, 2023. San Francisco, CA.
Disclosures
- Götberg reports consultant fee/honoraria/speaker's bureau fees from Boston Scientific Corporation, Abbott, Philips, and Medtronic.



Comments