Paclitaxel or Sirolimus: New PAD Studies Shed Light on Choices
Limus-based devices, rather than being a substitute for paclitaxel as they were once seen, are coming of age in peripheral disease.
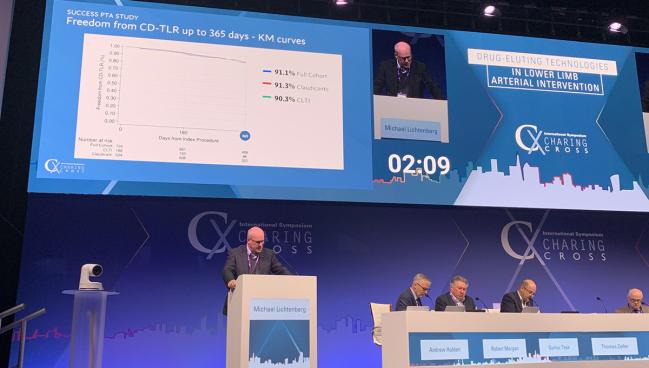
LONDON, England—While it’s not clear sirolimus-based devices will ever overtake the old workhorse paclitaxel, data from several studies presented here at the 2025 Charing Cross International Symposium show these technologies may have their place in the armamentarium of operators performing interventions for peripheral vascular disease.
In multiple presentations featuring patients with a wide range of disease complexities, there were hints for how sirolimus may differ therapeutically from paclitaxel and what role it may have once long-term efficacy and durability are known.
In the SUCCESS PTA trial, for instance, the sirolimus-eluting Selution SLR (Cordis) DCB was on par with established paclitaxel-coated balloons, including Ranger (Boston Scientific), In.Pact Admiral (Medtronic), and Passeo-18 (Biotronik), when it came to freedom from clinically driven TLR.
“These data continue to demonstrate the consistency of positive results across this new technology,” primary investigator Michael Lichtenberg, MD (Arnsberg Clinic, Germany), said in his presentation. “This study is proof that sirolimus works from a clinical context.”
SUCCESS PTA and SIRONA
SUCCESS PTA enrolled 723 patients from 27 sites in Europe, Asia, and South America. Nearly 40% had diabetes, 17% had renal failure/impairment, and 26% had chronic limb-threatening ischemia (CLTI; Rutherford class 4, 5, or 6). Mean lesion length was 128.4 mm, and 16% had a calcium grade of 4. Overall, 8% of patients had a below-the-knee (BTK) lesion.
The device success rate was 99%, and the procedural success rate was 98%. At 1 year, rates of freedom from clinically driven TLR were 91.1% in the full cohort (91.3% in claudication and 90.3% in CLTI).
In terms of safety, target lesion amputation rates were low at 2.2% (0.2% for claudication and 9.4% for CLTI).
Improvements in ankle-brachial index seen at 6 months were maintained at 12 months, Lichtenberg noted. The same was true for quality of life as measured by the EuroQol-5D. Follow-up is planned out to 5 years.
Commenting from the audience, George Adams, MD (University of North Carolina at Chapel Hill, Raleigh), said he was struck by the freedom from clinically driven TLR in the CLTI patients, noting they are “some of the highest numbers I have ever seen.”
Lichtenberg said while more research is needed to inform these results, they provide some inspiration for investigators to “put our efforts in” to understand if there is perhaps an enhanced response to sirolimus in the CLTI population. Currently, he is heading the SELUTION4BTK trial, which should help add more needed insights.
“This book chapter is definitely not closed, it is just opened and I think it’s important to learn here,” he said. According to Lichtenberg, the answer to understanding the role of sirolimus and paclitaxel may be combinations of strategies such as vessel preparation combined with the balloon and the most appropriate antiproliferative therapy for the patient being treated.
Last year, Ulf Teichgräber, MD, (University Hospital Jena, Germany), showed that another sirolimus balloon (MagicTouch; Concept Medical) was noninferior to paclitaxel drug-coated balloons (DCBs) in the SIRONA trial. Here, Teichgräber presented updated results from a deep dive into the clinical improvement (≥ 1 Rutherford category) at 12 months, demonstrating that the paclitaxel group fared better (P = 0.043). Conversely, pain-free walking distance was slightly better in the sirolimus group, but was not statistically significant.
HOPE Registry
More data from a CTLI population came from a presentation of HOPE registry data by Edward Choke, MBBS, PhD (Northern Heart Hospital, Penang, Malaysia). At 3 years, sirolimus-based DCB (MagicTouch) was associated with fewer BTK reinterventions compared with plain balloon angioplasty (P = 0.033).
“We think that this is because sirolimus is effectively inhibiting the neointimal hyperplasia that starts to occur from about 5 months on,” Choke said. The sirolimus-treated patients also saw better amputation-free survival and better overall survival in this highly comorbid real-world population.
With evidence at this stage that both drugs work, moderator Andrew Holden, MBChB (Auckland Hospital, New Zealand), wondered how clinicians will eventually choose which to use in their PAD interventions.
If you have free choice, then you can differentiate lesion characteristics [and] outflow characteristics regarding device selection. Thomas Zeller
Panelist Thomas Zeller, MD, PhD (Universitäts-Herzzentrum Freiburg-Bad Krozingen, Germany), said as with most things, it may come down to cost and what is available to the operator at their institution.
“Of course, if you have free choice, then you can differentiate lesion characteristics [and] outflow characteristics regarding device selection,” he said. “For example, if you have a bad outflow situation, you should probably not use long paclitaxel crystalline-coated devices. You should use a limus-coated balloon. If it's a simple [lesion], then you can go with paclitaxel.”
Lichtenberg noted that paclitaxel DCBs have progressed with each generation and have fewer issues like cracking or flaking of the drug on the balloon than were seen in the past.
“This is bringing us to a new level as we have more safety data now for modern [devices], including of course paclitaxel and sirolimus,” he said. “I think this should drive how we do the interventions.”
Paclitaxel Mortality No Longer the Main Question
After years of being the standard drug for DCBs in PAD treatment, a safety signal for paclitaxel emerged in 2018 from a meta-analysis that led to a period where paclitaxel-based devices for peripheral interventions were taken out of use around the world. Ultimately, the mortality signal was refuted by numerous datasets and the US Food and Drug Administration removed black box warnings from paclitaxel DCBs in 2023.
While there was intense interest in sirolimus devices as alternatives during the paclitaxel moratorium, Brian DeRubertis, MD (NewYorkPresbyterian/Weill Cornell Medical Center and Cornell University Medical College, New York, NY), asked from the audience if researchers have finally put the paclitaxel safety questions behind them.
In response, Teichgräber said the question that remains now when considering sirolimus versus paclitaxel is no longer one of mortality, noting there are concerns about things such as vessel wall degradation from paclitaxel use. It’s uncertain yet if sirolimus is a less aggressive drug that yields similar treatment effects, he said.
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Multiple presentations. Presented at: Charing X Symposium. April 23, 2025. London, England.





Comments