PARTHENOPE Adds to Polymer-Free Stent Data, But Raises ST Questions
The uptick in stent thrombosis with this amphilimus-eluting stent means it’s unlikely US regulators will approve it, says Ajay Kirtane.
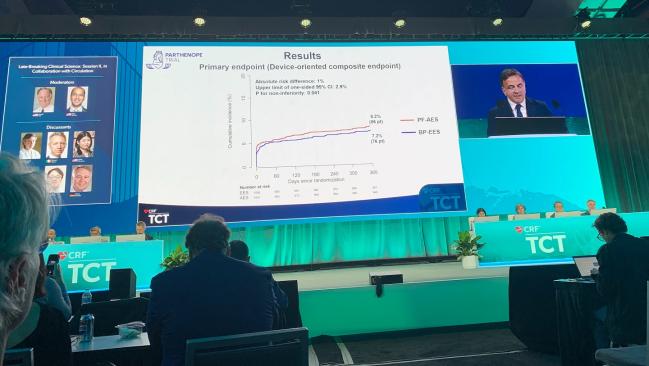
SAN FRANCISCO, CA—At 12 months, a polymer-free, amphilimus-eluting stent was noninferior to a biodegradable-polymer DES in all-comers undergoing PCI, according to results of the PARTHENOPE trial. However, there was an excess of stent thrombosis in the polymer-free group.
“There were no differences in the components of the primary endpoint [of] cardiovascular death, target-vessel MI, and clinically indicated TLR between the two study devices,” said Giovanni Esposito, MD, PhD (University of Naples Federico II, Italy), in a late-breaking clinical science presentation last week at TCT 2023.
The thin-strut, polymer-free Cre8 stent (Alvimedica) stent features a unique design with reservoirs that control the release of the drug such that 50% is gone within 18 days, 65% to 70% within 30 days, and complete drug elution within 90 days. The device has previously been tested against a permanent-polymer zotarolimus-eluting stent (ZES) in an “all-comers” population in the ReCre8 trial and in a diabetic population in the SUGAR trial. In both of those trials, the polymer-free device was noninferior to ZES.
Noting that “there is an unmet need for more data in this area,” discussant Robert Byrne, MBBCh, PhD (Mater Private Network, Dublin, Ireland), said the polymer-free stent has emerged as a promising investigational device, the trial seems representative of clinical practice, but the signal of harm is eye-catching.
“There was this increase in definite or probable stent thrombosis and it’s difficult to see past it,” Byrne added. “I think this probably is going to define the result of this trial, unfortunately. It’s not a benign event, there’s a 1% increase in cardiovascular mortality in the trial and I suppose there really is only one question: is there any explanation from looking at the data as to why this might have been the case? Because if I look back at the previous trials with this stent, I didn’t see a signal of stent thrombosis.”
Noninferiority Criteria Met
PARTHENOPE was conducted at 14 centers in Italy and enrolled all-comers patients (mean age 64; 23% women, 37% STEMI), with target lesion lengths between 2.25 and 4.5 mm.
Patients were randomized to receive either the Cre8 stent (n = 1,051) or the Synergy bioabsorbable polymer everolimus-eluting stent (Boston Scientific; n = 1,056), and further randomized to either personalized dual antiplatelet therapy, based on baseline DAPT score, or to standard DAPT, which was 12 months of dual therapy. Postdilation was performed in 62% of the polymer-free group and in 57% in the bioabsorbable-polymer group.
There was this increase in definite or probable stent thrombosis and it’s difficult to see past it. Robert Byrne
The device-oriented composite endpoint (CV death, MI not clearly attributable to a non-target vessel, or clinically driven TLR within 1 year) occurred at a rate of 8.2% in the polymer-free group and 7.2% in the biodegradable-polymer group, meeting criteria for noninferiority (P for noninferiority = 0.04), but not for superiority (P = 0.39). The results were similar for the individual components of the primary endpoint and consistent across multiple subgroups that included age, gender, diabetes, STEMI, and vessel size.
None of the secondary endpoints were significantly different between the two groups with the exception of stent thrombosis. Eleven patients in the polymer-free group had definite or probable stent thrombosis versus three patients in bioabsorbable-polymer stent group (P = 0.04). Most of these were early definite stent thrombosis that occurred in the first 30 days. According to Esposito, after adjustment for multiple comparisons, the difference was not statistically significant.
Moreover, he added, none of the stent thrombosis events led to CV death, but he acknowledged that more analysis of this endpoint is needed.
“Even though they don’t relate to cardiovascular mortality, a stent thrombotic event is not a good event to have,” said session moderator Ajay Kirtane, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY).
Speaking with TCTMD, Kirtane said that the earlier, “promising” studies typically persuade US operators that they “would like to have access to the technology, so it’s worth going through the FDA approval process to get it.” However, he continued, “after seeing these data, I think the likelihood of that happening here is low and the desire to add yet another drug-eluting stent to our armamentarium on the basis of that data is unlikely.”
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Esposito G. Polymer-free amphilimus-eluting stents vs. biodegradable-polymer everolimus-eluting stents in all-comers undergoing PCI: 1-year results of the PARTHENOPE trial. Presented at: TCT 2023. October 25, 2023. San Francisco, CA.
Disclosures
- Esposito reports grants and research support to his institution from Alvimedica, Boston Scientific, and Medtronic; and consultant fees/honoraria from Amgen, Boehringer Ingelheim, Edwards Lifesciences, Novartis, Amarin, and Sanofi.





Comments