PARTNER 3: TAVR Now Noninferior to Surgery at 2 Years in Low-Risk Patients
Catch-up seen in outcomes may be due to chance or something more worrisome, but it’s too early to say, said Michael Mack.

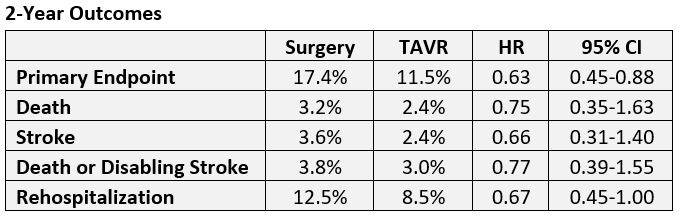
Two-year results in patients with aortic stenosis at low risk for surgery continue to show a numerical benefit of TAVR over surgery with regard to the study’s primary endpoint of death, stroke, or cardiovascular rehospitalization, but the lead has narrowed.
An uptick in deaths and strokes in the TAVR arm appear to be closing the gap, according to new numbers from PARTNER 3.
As reported by TCTMD, 1-year results of PARTNER 3 showed superiority of TAVR over surgery for the combined primary endpoint, with results so dramatic—a 7.1% difference translating into a 48% treatment effect—as to surprise even the investigators. This update shows a 5.9% difference translating to a 37% treatment effect at 2 years, still meeting noninferiority criteria (P = 0.007). Investigators did not test for superiority at 2 years.
“People are going to take away from [the study] what they want to take away from it,” Michael Mack, MD (Baylor Scott & White Heart Hospital, Plano, TX), who presented the 2-year PARTNER 3 data today during the virtual American College of Cardiology 2020 Scientific Session, told TCTMD. Some people might “appropriately” note that TAVR at this point is noninferior to surgery even with a narrower gap in outcomes, he said. “And then the others are going to look at the curves and see them get closer and say, ‘Aha! You know what we're beginning to see here? That at some point in time the lines will cross and be in favor of surgery.’ But it's all total speculation.”
Results at 2 Years
PARTNER 3 included 1,000 patients with an STS Predicted Risk of Mortality of less than 4% (mean STS-PROM score 1.9%) treated with the balloon-expandable Sapien 3 transcatheter heart valve (Edwards Lifesciences) or surgery at 71 centers. The average age of those treated was 73.4 years, and nearly 70% of patients were men.
At 2 years, TAVR maintained superiority for the primary endpoint but not for the individual components, with the exception of rehospitalization.
The main reason for rehospitalization in both groups was congestive heart failure. As for neurological events, one nondisabling stroke occurred in surgically treated patients between years 1 and 2, whereas six TAVR patients had neurological events, three of which were disabling strokes and three nondisabling.
As at 1 year, the incidence of new-onset A-fib was lower in the TAVR arm (7.9% vs 41.8%) and the incidence of new LBBB was higher (24.4% vs 9.4%; both P < 0.001). However, valve thrombosis events as defined by VARC 2 criteria had occurred more often in the TAVR arm at 2 years (2.6% vs 0.7%; P = 0.02).
The echocardiography findings show no change in mean gradient (13.6 vs 11.8 mm Hg; P < 0.001), aortic valve area (1.7 vs 1.7 cm2; P = 0.69), or paravalvular regurgitation (P < 0.001) between TAVR and surgery at 2 years.
Serendipity or a Signal?
As for why there may have been some catch-up in the TAVR arm between years 1 and 2, Mack said “it's impossible to say.”
The events that happened in the TAVR arm specifically should not be ignored, Mack continued, “but attaching real significance to [them] at this point that would influence clinical decision-making I think is an overreach.”
The main take-home message from the data at this point, according to Mack, is “that there are two good options for low-risk patients and that there should be shared decision-making with the patient as to which option is best for them.” He emphasized that PARTNER 3 was not an all-comers trial, “so if you put it in that framework and have patients who were studied in the trial, both options are good options based upon 2-year data. However, extending the findings of this trial to the population outside of that study is a bridge too far.”
In discussion following the presentation, Howard Herrmann, MD (University of Pennsylvania Perelman School of Medicine, Philadelphia), said his biggest concern in the data “is the increase in stroke and valve thrombosis both numerically and relative to SAVR between years 1 and 2 despite the overall lack of difference in the primary endpoint between TAVR and SAVR at 2 years.” He asked how many of the study patients had CT confirmation of either valve thrombosis or leaflet thickening and whether any of these events related to A-fib.
“All patients that had a demonstration of valve thrombosis did have imaging confirmation of the valve thrombosis either by CT or by echo,” Mack replied. “Of the stroke patients, the six that occurred, there was one patient that had preexisting A-fib and one patient with new onset A-fib. So only two of the patients were possibly related to A-fib. The remainder were not.”
Hermann also wanted to know which clinical findings Mack thought clinicians should be looking out for in order to prompt an imaging study and potential prescription of an oral anticoagulant. Also, he asked: “Given the risk of oral anticoagulant therapy with DAPT that we've seen in today's session and other studies, should we be using vitamin k antagonists preferentially in this situation?”
A change in hemodynamics, increase in valve gradient, rise in paravalvular leak, or change in symptoms should prompt an imaging study, Mack said. “Only with confirmation of valve thrombosis on imaging study should anticoagulation be considered,” he advised. “Oral anticoagulation is not benign. Of the clinical events associated with valve thrombosis here, two of the six were related to anticoagulation, so the decision I think should be based on an individual patient’s valve thrombosis severity as well as the risk of anticoagulation. Regarding whether patients should receive warfarin or a [non-vitamin K antagonist oral anticoagulant], I don’t think we have any evidence that there’s any benefit to anything other than warfarin in the current time, again based on other trials.”
Mack, in response, explained that the researchers used VARC 2 definitions for valve thrombosis, which use an elevated gradient of > 20 mm Hg as a cutoff. “We also have looked using VARC 3 definitions of the trial, and using current VARC 3 criteria for structural valve deterioration and bioprosthetic valve failure the incidence is very low in both arms and no difference between TAVR and surgery,” he added. Mack told TCTMD that these findings will likely be presented later this year.
Hung also asked about durability, which is “obviously a critical issue” for low-risk patients who are often younger. “TAVR leaflets may be subject to increased mechanical stresses due do the design and needing to be crimped inside a sheath,” she said. “Can you comment on whether the increased valve thrombosis observed in TAVR was potentially related to early structural valve degeneration?”
Mack stressed the limited ability of 2-year follow-up to show anything definite about durability. “Having said that, looking specifically at structural valve deterioration using the new criteria, we do not see an increased incidence of TAVR compared with surgery,” he said. “The incidence is very low, in the 1% range, in both TAVR and surgery using current definitions of structural valve deterioration. Again, I think it’s way too early to be expected to see a signal, but I think it is somewhat comforting at this point that there’s no signal of early structural valve deterioration.”
These patients will be followed out to 10 years, Mack said, at which point any problems with structural valve deterioration will become apparent.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Mack MJ. Two-year clinical and echocardiographic outcomes from the PARTNER 3 low-risk randomized trial. Presented at: ACC 2020. March 29, 2020.
Disclosures
- Mack reports receiving research support from Abbott, Edwards Lifesciences, Gore, and Medtronic and serving as a trial co-PI or study chair for Abbott, Edwards Lifesciences, and Medtronic.


Comments