PARTNER: All-Cause, CV Mortality Lower in Patients Treated with TAVI vs. Standard Therapy at 1 Year
WASHINGTON, DC—New randomized clinical trial data demonstrate statistically significant reductions in all-cause and CV mortality with transcatheter aortic valve implantation compared with standard therapy in patients who are not candidates for surgical therapy, according to data presented at TCT 2010.
“Although patient selection, operator skills and technology have improved, all previous transcatheter aortic valve implantation (TAVI) studies have been observational registries, without standardization of endpoint definitions,” TCT Course Director Martin B. Leon, MD, of Columbia University Medical Center, New York, said in his presentation.
The PARTNER investigators randomized 358 patients with severe aortic stenosis and cardiac symptoms who were unable to undergo surgery. They then assessed the safety and effectiveness of TAVI (n=179) vs. standard therapy (n=179).
Primary, co-primary endpoints
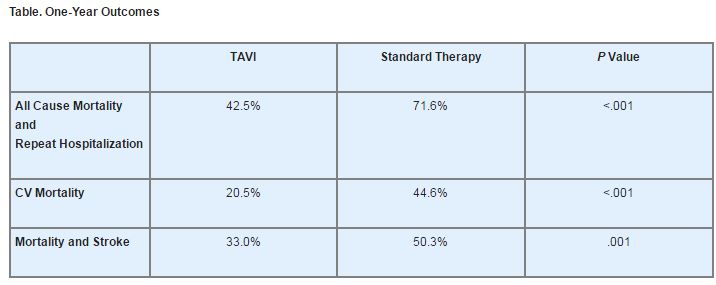
At 30 days, all-cause mortality was numerically higher in the TAVI group (5.0% vs. 2.8%, P=.41), as was CV mortality (4.5% vs. 1.7%, P=.22). At 1 year, however, patients who underwent TAVI demonstrated a significant all-cause mortality advantage vs. those who had received standard therapy.
The co-primary composite endpoint of all-cause mortality and repeat hospitalization also favored the TAVI arm, as did several other 1-year outcomes (see Table).
The number needed to treat to prevent the co-primary endpoint was 3.4, while the numbers needed to treat for CV mortality and the composite of mortality and stroke were 4.1 and 5.5, respectively. Furthermore, the 6-minute walking distance improved in both arms; however, only the improvement in the TAVI arm (baseline, 73 m vs. 1 year, 120 m; P=.002) was statistically significant.
Major vascular complications were higher at 30 days for TAVI vs. standard therapy (16.2% vs. 1.1%, P<.0001), as was the frequency of major bleeding episodes (16.8% vs. 3.9%, P<.0001). The frequency of major strokes also tended to be higher at 30 days in the TAVI arm vs. standard therapy (5.0% vs. 1.1%, P=.06).
Clinical implications
Leon emphasized that TAVI should be the new standard of care for patients with aortic stenosis who are not suitable candidates for surgery.
“Next generation devices may help to reduce the frequency of procedure-related complications in the future,” he said. “The ultimate value of TAVI will depend on careful assessment of bioprosthetic valve durability, which will mandate obligatory long-term clinical and echocardiographic follow-up of all TAVI patients.”
Following the presentation, Patrick T. O’Gara, MD, of Harvard Medical School, Boston, provided additional perspective on what the PARTNER trial means for clinical practice.
“Although the number of patients reported in this cohort is relatively small, the trial itself reestablishes the importance of the randomized, controlled clinical trial and our understanding of both the efficacy and the safety of invasive procedures for the treatment of valvular heart disease,” he said. “I think as most of us would agree that the field of valvular heart disease has relied too heavily on single-institutional observational studies or registries that have, as a result, left us with a level-of-evidence-C recommendations for patient management.”
O’Gara also noted that the PARTNER data represent the first randomized, controlled clinical data of their kind, even though “more than 15,000 of these procedures have been performed worldwide.”
Disclosures
- Dr. Leon reports serving on the scientific advisory boards for Edwards Lifesciences, Medtronic and Symetis. He also reports an equity relationship with Sadra.
- Dr. O’Gara reports no relevant conflicts of interest.
Comments