Patch May Prevail Where Prior Wearable Defibrillators Faltered: Jewel IDE Study
A novel solution, already cleared in Europe, aims to increase patient comfort and convenience while minimizing false alarms.
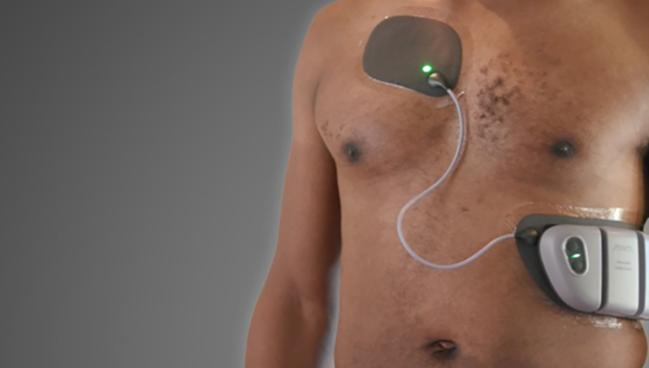
Photo Credit: Element Science
A creative approach to wearable cardioverter-defibrillators (WCDs)—in the form of a water-resistant patch—appears to be both safe and effective in patients with ventricular arrhythmias that put them at increased risk of sudden cardiac death, according to results from the Jewel Investigational Device Exemption (IDE) Study.
Jewel (Element Science), which was cleared for marketing in Europe earlier this year but is not yet sold in the United States, employs a water-resistant patch equipped with a machine-learning algorithm for detecting and treating sustained, shockable rhythms while minimizing false alarms. The product consists of a monitoring and defibrillation unit as well as an 8-day patch and battery unit that are worn continuously until they need to be replaced with a new set.
Its design may help overcome issues with compliance that have dogged earlier WCDs, which have been on the market for years but require patients to wear a vest.
Javed Butler, MD (Baylor Scott and White Research Institute, Dallas, TX, and University of Mississippi, Jackson), senior author of the new paper, said the Jewel WCD fills a niche need for patients who need temporary protection against sudden cardiac arrest.
“But can you have something which the patients will feel more easy to wear, including during physical activity and when they take a shower and all that kind of stuff—to just be compatible with their lifestyle? And I think in that aspect, the results were very encouraging,” he told TCTMD, citing the rarity of skin reactions with the patch and appropriateness of shocks in the Jewel IDE Study.
“This is a ‘stick it and forget it’ sort of a thing. It's an adhesive patch and you just put it on, so you're not actively wearing or taking something off,” Butler explained.
The problem with prior WCDs, he told TCTMD, is that even when the data show they work well when worn, cardiac deaths can occur when patients aren’t wearing them as prescribed.
In 2016, the American Heart Association issued a scientific statement that outlined where the devices would be most useful but also highlighted that evidence was still sparse. In the years that followed, the first randomized trial of a WCD, which focused on the LifeVest (Zoll), failed to meet its primary endpoint of a decrease in arrhythmic death but did show a significant drop in total death. The finding raised hope among some that WCDs might prove useful in select patients, provided that the devices are worn consistently enough to provide protection.
Led by John Hummel, MD (The Ohio State University Wexner Medical Center, Columbus), this latest study was published online this week in the Journal of the American College of Cardiology.
‘Stick It and Forget It’
For the single-arm Jewel IDE study, conducted at 30 US sites, the researchers analyzed data on 305 patients (mean age 57.9 years; 30.2% female; 27.9% nonwhite) at risk for sudden cardiac arrest due to ventricular tachycardia/ventricular fibrillation. All either refused or were not candidates for an implantable cardioverter-defibrillator (ICD); the most common indications for the WCD were nonischemic cardiomyopathy (35.1%) and temporary contraindication to ICD (26.2%).
Participants continued in the study for a median of 59.3 days, and the median wear time was 23.5 hours per day. Ultimately, 163 patients (53.4%) exited for clinically indicated reasons, including 50.2% who left when a WCD was no longer indicated and 12.1% who decided to leave due to skin-related concerns.
Cutaneous adverse device events occurred in 42.6% of patients, though 70.9% of those were mild and most consisted of a rash. None was severe. The events were considered clinically significant if they resulted in a physician withdrawing a patient from the study—the rate of that primary safety endpoint was 2.3%, falling within the prespecified threshold of < 15%.
Additionally, there were no device-related deaths or severe adverse events.
The study also met its primary effectiveness endpoint, defined as less than two inappropriate shocks per 100 patient-months, coming in at 0.36 inappropriate shocks per 100 patient-months. Three-quarters of those false alarms were seen in five patients.
In total, nine patients had 11 shocks, nine of which were appropriate. Out of those nine, eight were successful with a single shock. Most patients (61.7%) had no alarms during the study, and 88.9% had fewer than one alarm per day.
As for downsides, Butler said that a “perpetual issue” with any new device is that its commercial costs are unknown before it hits the market. With Jewel, “every 8 days you have to change the patch,” he noted. That said, it appears to work very well and future studies can delve into the financial trade-offs, Butler observed. “Whether or not it passes the muster of cost-effectiveness, we'll see, and we'll see what people are going to use.”
He suggested that other future trials could look into subpopulations like patients with new-onset heart failure or who have a drop in ejection fraction after MI.
Patient, Physician Acceptance of WCDs
Emile G. Daoud, MD (TriHealth Heart and Vascular Institute, Cincinnati, OH), in an accompanying editorial, notes that the US Food and Drug Administration has approved not only LifeVest but also the Assure WCD (Kestra Medical Technologies) as bridge therapies for automatic defibrillation during a high-risk period before the lasting treatment is chosen, such as LVEF recovery, an ICD, or heart transplant.
That said, issues related to “patient acceptance and compliance” have been barriers to adoption, he points out. Despite the evidence that Jewel is “certainly a successful” therapy, these barriers are likely to remain in place, Daoud adds. “Perhaps one reason for reduced patient acceptance of this life-saving therapy is the absence of a perceived benefit. As a prophylactic device, the patient does not experience an improvement in physical health and thus positive feedback for the WCD is absent.”
Continuous patient education seems helpful as a means to overcome this, he suggests. “Ongoing counseling could even be automated via daily texts to encourage patients to use their WCD, because, of course, without patient acceptance, no WCD technology will stick.”
According to Butler, there are two levels at which adoption can be hindered: among physicians deciding whether their patient requires temporary protection from sudden cardiac arrest and among patients deciding whether to wear their cardioverter-defibrillator. “For both, there is substantial variability,” he said. “There are physicians who are very aggressive and they want to do everything, and there are other physicians who are saying, ‘Well, you know, most of the patients are going to do okay anyway, so why do we need this?’”
He continued: “And then . . . there are some patients who are really scared and they're going to say, ‘I don't care how uncomfortable it is, it's important as a medically recommended therapy and I'll do it,’ and other patients with any discomfort will just say, ‘I'm not doing it.’”
Physicians currently face data that’s “relatively thin” for any of the WCDs, Butler said. “Therefore, I think the uptake, in my opinion, is more modest for these devices than what the promise [would suggest].” Only randomized controlled trials can quantify that promise, he added.
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Hummel J, Houmsse M, Tomassoni G, et al. A patch wearable cardioverter-defibrillator for patients at risk of sudden cardiac arrest. J Am Coll Cardiol. 2024;84:525-536.
Daoud EG. Patch wearable defibrillator: excellent therapy, but patient acceptance is a sticky issue. J Am Coll Cardiol. 2024;84:537-539.
Disclosures
- The Jewel IDE trial was conducted with support from Element Science. The sponsor was responsible for site selection, data monitoring, and overall clinical trial management. All statistical analyses were performed by an independent statistician.
- Butler has received consulting fees from Abbott, American Regent, Amgen, Applied Therapeutic, AskBio, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardiac Dimension, Cardiocell, Cardior, CSL Bearing, CVRx, Cytokinetics, Daxor, Edwards, Element Science, Faraday, Foundry, G3P, Innolife, Impulse Dynamics, Imbria, Inventiva, Ionis, Lexicon, Lilly, LivaNova, Janssen, Medtronic, Merck, Occlutech, Owkin, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Pharmain, Pfizer, Prolaio, Regeneron, Renibus, Roche, Salamandra, Sanofi, SC Pharma, Secretome, Sequana, SQ Innovation, Tenex, Tricog, Ultromics, Vifor, and Zoll.
- Hummel has received consulting fees from Medtronic, Volta Medical, S4 Medical, Abbott Medical, and Element Science.
- Daoud reports no relevant conflicts of interest.




Comments