Placebo-Controlled ORBITA-2 Shows PCI Eases Symptoms in Stable Angina
(UPDATED) In patients not taking antianginal meds, PCI clearly alleviated some—but not all—symptoms. What does that mean in practice?
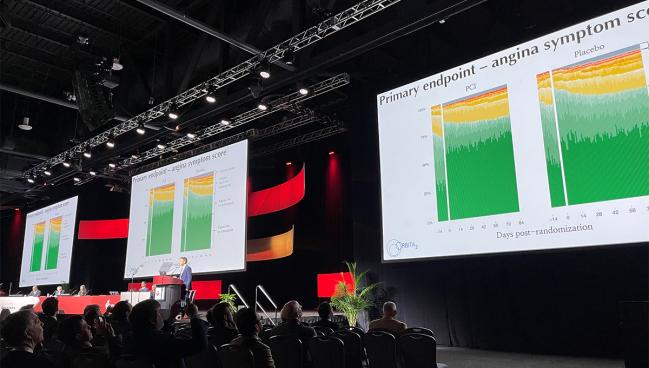
PHILADELPHIA, PA—More than 6 years since the placebo-controlled ORBITA trial set the interventional cardiology galaxy whirling, ORBITA-2, attempting to isolate the treatment effects of PCI alone, showed that an invasive strategy with drug-eluting stents does in fact reduce symptoms in patients with stable angina.
The study, which was presented today as a late-breaking clinical trial at the 2023 American Heart Association (AHA) Scientific Sessions and published simultaneously in the New England Journal of Medicine, found that PCI significantly reduced the anginal symptom score—a benefit that appeared driven by a reduction in the number of daily angina episodes—when compared with patients undergoing a placebo procedure.
“What we find here is that angioplasty is more effective than a placebo procedure,” senior investigator Rasha Al-Lamee, MBBS, PhD (Imperial College London, England), told TCTMD. “It's still less effective than unblinded angioplasty in previous trials, and it still leaves 59% of patients with symptoms, but it definitely has an effect.”
Investigators say that only by eliminating the use of guideline-directed medication as a precondition for PCI could they test its treatment effects on angina symptoms. In a way, ORBITA and ORBITA-2 mimic the renal denervation trials in that they tested the effects of PCI both on and off optimal medical therapy.
“I think the upshot, or the final message of this trial, is that you now have two pathways with PCI which have both been tested in a blinded, placebo-controlled way,” said Al-Lamee. “ORBITA told us that the effect of PCI on top of guideline-directed antianginal therapy is much smaller than we might expect. However, the effect of PCI as an up-front procedure may be where you see the greatest benefit.”
Speaking with the media, Martin Leon, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY), called ORBITA-2 a remarkable trial, noting that his surgical colleagues have long asked him whether PCI actually works. “Now I can say with confidence with a placebo-controlled trial that PCI certainly does have an impact,” he said.
Roxana Mehran, MD (Icahn School of Medicine at Mount Sinai, New York, NY), who moderated the late-breaking trial session, had a similar take on the data. “PCI works, ladies and gentleman,” she said to a laugh from the AHA audience. “It will reduce angina.”
To TCTMD, Robert Yeh, MD (Beth Israel Deaconess Medical Center, Boston, MA), who wasn’t involved in the study, said the finding that PCI improved patient symptoms wasn’t a surprise to physicians who take care of patients with angina, noting that when treatment—whether PCI or medical therapy—is offered to patients with severe enough symptoms there’s likely going to be a benefit. In ORBITA-2, patients were quite symptomatic and weren’t taking antianginals at the time of randomization as part of the study protocol, “so to see symptomatic improvement would have been expected,” he said.
In the original ORBITA study, after the aggressive treatment protocol, total angina burden was more modest so it may have been more difficult to see a treatment benefit with PCI, although there were clearly signals of anginal improvement with PCI over meds, even in that trial, Yeh said.
What we find here is that angioplasty is more effective than a placebo procedure. Rasha Al-Lamee
Similarly, Pinak Shah, MD (Brigham and Women’s Hospital, Boston, MA), said the beneficial effects of PCI weren’t unexpected. “We’ve all believed that PCI should help symptoms,” he told TCTMD. “We also know that medications can help take care of symptoms in patients with stable coronary disease. What we hadn’t tested—and I give this team a lot of credit for looking at this—was whether or not there's an effect of PCI that's independent of medications. These [placebo-controlled] studies are not easy to do.”
William Boden, MD (Boston University School of Medicine/VA New England Healthcare System, MA), who led the COURAGE trial that showed PCI didn’t reduce the risk of death, MI, or other major CVD events when added to medical therapy in stable patients, called the symptomatic improvement in ORBITA-2 relatively “modest.” Like others, he believes a beneficial effect was expected given that the trial included patients with significant angina, obstructive coronary disease, and physiologic confirmation of limited flow.
“If you stent the artery successfully, you should expect these patients to become symptom free,” Boden told TCTMD. “We’ve known for 40 years that’s what PCI does—it relieves angina—and I think they demonstrated that pretty conclusively in a very elegant way because they’ve obviously controlled for a lot of factors here.”
Boden praised the research group, which included lead investigator Christopher Rajkumar, MBBS (Imperial College London), noting that that ORBITA-2 was an extremely challenging, multicenter study. “The way they conducted it, they deserve enormous credit.”
Second Placebo-Controlled Trial in Orbit
In 2017, the ORBITA investigators left many cardiologists incredulous when they showed that coronary revascularization with PCI was associated with a significant placebo effect, prompting the full range of reactions, including calls for an immediate change to practice and guidelines. In that study, PCI for single-vessel disease in 200 patients with stable angina was no better than a placebo procedure when it came to improvements in exercise capacity and symptoms. Two years after ORBITA, the ISCHEMIA investigators went on to show that an invasive strategy with PCI or CABG surgery didn’t reduce the risk of hard clinical events compared with a conservative strategy using medications.
In the current US and European guidelines, both medication and PCI play a prominent role in treating symptomatic CAD patients, with optimized antianginal therapy considered the frontline therapy (class 1 indication) and PCI reserved for those who fail antianginal therapy (also a class 1 indication).
“I always felt that one of the most likely explanations for the surprising result of ORBITA was that we had used the guideline-directed approach,” said Al-Lamee. “They were on an average of three antianginals after they finished the medical optimization phase, which is far more than we usually see in clinical practice. Those who remained symptomatic despite antianginal therapy might have less to gain with angioplasty because we were now selecting a population of patients who may have a variety of different reasons for their chest pain.”
ORBITA-2 isolated the treatment effect of PCI but differed from ORBITA in other ways: it was 12 as opposed 6 weeks long, the primary endpoint was an angina symptom score (versus treadmill exercise time), and the patient mix included those with multivessel CAD with documented evidence of ischemia in at least one cardiac territory.
In total, 439 patients with angina or an anginal equivalent were enrolled in the prerandomization symptom-assessment phase. During this 2-week stage, all antianginal medications were stopped and patients were asked to report daily symptoms via a smartphone application. Only those with at least one angina episode during the assessment phase proceeded to randomization.
We’ve all believed that PCI should help symptoms. Pinak Shah
Patients entering the trial underwent coronary angiography with auditory isolation using over-the-ear headphones and invasive physiological assessments. If randomized to PCI, patients underwent mandatory angiographic and physiological complete revascularization of target vessels; the use of intravascular imaging was recommended.
In the end, 301 patients (mean age 64 years; 79% male) were randomized between PCI or a placebo procedure. At enrollment, 96% had CCS class II or III symptoms and patients were taking a median of one antianginal medications before being stopped (the equivalent of two standardized antianginal units). Eighty percent of patients had single-vessel disease, while 17.0% and 2.3% had two- and three-vessel disease, respectively.
Improvement in Angina Symptom Score
At 12 weeks, the angina symptom score—an ordinal outcome scale comprised of the number of angina episodes reported daily, units of antianginal medications, and high-level unblinding overrides for intolerable angina, ACS, or death—was significantly lower among patients who underwent PCI than those treated with placebo (2.9 vs 5.6; P < 0.001). The benefit was mostly driven by a reduction in the number of daily angina episodes (0.3 vs 0.7; OR 3.44; 95% CI 2.0-5.9), with no significant difference seen between groups in the number of patients who received antianginal medications (0.2 vs 0.3 units; OR 1.21; 95% CI 0.70-2.10).
Secondary endpoints also improved with PCI. For example, treadmill exercise time was greater with PCI (700.9 vs 641.4 seconds) and the physician-assessed CCS class was lower (0.9 vs 1.7). The 60-second improvement in exercise time is roughly what patients could expect from taking one antianginal agent, said Al-Lamee. Additionally, there were improvements in the Seattle Angina Questionnaire (SAQ) frequency, physical limitation, quality of life and freedom from angina scores as well as improvements in quality of life assessed with the EuroQOL questionnaire.
Unblinding for intolerable angina occurred in just one patient in the placebo group and none in the PCI arm. ACS occurred in four patients randomized to PCI and six in the placebo arm. In a test of blinding, investigators found that both patients and staff were effectively unaware of treatments both prior to discharge and at 12 weeks.
Connie Hess, MD (University of Colorado Medicine, Aurora), who discussed the trial following the late-breaking clinical trial presentation, said “the results of ORBITA-2 are clear.” In addition to symptomatic improvement, few adverse events were reported overall, she said. In terms of limitations, Hess pointed out that the majority of patients were male: “We know that patients who are female experience angina differently and may have different contributory mechanisms, including microvascular dysfunction or vasospasm.”
In looking at the results, Yeh praised the trial as an important scientific experiment designed to quantify the treatment effect of PCI alone. He noted, however, that the angina symptom score is a newly developed endpoint, so it’s difficult to spell out the clinical interpretation of the observed differences. For him, the fact that PCI-treated patients were roughly three times more likely to be free from angina 12 weeks after revascularization was a meaningful benefit that would be understood by patients and their families.
Standard practice with stable CAD is to first treat medically. William Boden
Similar to Al-Lamee, several cardiologists homed in on the extent of residual angina. Yeh said that while nearly 60% of patients continued to have symptoms after PCI, this is a diagnostic problem as opposed to a therapeutic one. “Ideally, physicians would be able to identify whether and which coronary blockages were responsible for a patient’s symptoms,” he said. “But right now, there’s no test that tells us whether a patient’s symptoms are really attributable to epicardial coronary disease.”
More accurately identifying which stable angina patients will benefit from PCI, as well as from medical therapy, will continue to be an important goal for researchers, he said. Leon agreed, saying the mechanism underpinning residual angina after successful PCI is “curious” and needs to be explored further.
PCI Up Front? Not So Fast
Al-Lamee said that in designing the primary endpoint, which was based on discussions with patients and physicians and ranks medication burden ahead of angina symptoms, investigators learned patients would prefer to be slightly more symptomatic while taking less medicine than to have fewer symptoms while on more medication. For many, side effects are a big issue, particularly fatigue, dizziness, headaches, and erectile dysfunction. There is also the burden of taking multiple medications per day, particularly if they have other conditions.
“I think what we have now is the ability to give two sets of options to our patients with their own associated benefits, risks, and costs,” said Al-Lamee. “And with the data that we have from ISCHEMIA and COURAGE, we know we don’t have to rush. There’s enough time to talk to them and work out what suits them and what we think would be the best approach.”
Whether PCI might be best when deployed as an up-front therapy, before patients have tried and failed on antianginals—that’s an “interesting” concept, said Yeh, but “not really the hypothesis that was tested in the trial.” While pill burden and polypharmacy are a real issue for older patients and side effects can be burdensome to many, “these are safe, inexpensive medications compared with an invasive procedure that is not without risk. In most cases, I still believe medications should certainly be tried first,” said Yeh.
Boden agreed unequivocally. “Standard practice with stable CAD is to first treat medically,” he said. “One antianginal is not optimal antianginal therapy.”
In 2022, Boden, along with several coauthors, including Al-Lamee, published a viewpoint laying out how physicians should go about using medication—and for how long—before referring patients to revascularization. They recommend an empiric trial of 2-3 months of antianginal intensification (assuming the patient agrees and can tolerate it) before declaring medication a failure.
Right now, there’s no test that tells us whether a patient’s symptoms are really attributable to epicardial coronary disease. Robert Yeh
“In my view, we do not do a good job of allowing medical therapy an opportunity to work,” he said. Boden stressed, though, that a patient-centered approach is important and healthcare teams need to listen to what patients are asking. “If they’re telling you they’re averse to taking medication, they don’t want side effects, and they’d rather have a procedure to improve their quality of life, then I think that’s appropriate,” he said.
Shah also agrees with the patient-centered approach, noting there is merit in treating select patients with PCI earlier for symptom relief if the procedure can be performed safely.
“What I take away from all this is that if I have a patient who has relatively straightforward disease that can be treated with PCI, nothing terribly complicated, certainly medications will help manage that patient's symptoms,” said Shah. “If they fail, I can take the approach of doing a PCI. But medications themselves are also a quality-of-life issue for a lot of people. If they can just get a PCI that's relatively safe and not have to worry about those extra medications, then I think we're fully justified in doing that.”
Real-world Practice
Speaking to the press, Leon said he believes the ORBITA-2 results will challenge the existing clinical guidelines that give precedence to medical therapy over PCI. “This trial is going to raise many questions going forward,” he predicted.
To TCTMD, Al-Lamee said ORBITA-2 reflects the types of patients sent for PCI in the real world, noting the vast majority are currently undergoing revascularization with minimal or no antianginal agents. “If the guidelines do not reflect what we’re practicing out there in clinical practice, then we need to understand the benefits of angioplasty in the real-world,” she said.
Shah agreed, noting that he sometimes encounters patients referred for PCI who might benefit from an additional nudge with medications.
This trial is going to raise many questions going forward. Martin Leon
“We often don't meet these patients until the time of the procedure,” he said. “When the patient is there ready, and they’ve been told you'll get a stent and you'll be fine, that’s a hard train to stop sometimes. But in some instances, after reviewing the case, I’ll think PCI is not an appropriate thing to do. We’ll call the [referring] physician and say, ‘Hey, listen, we haven't really gotten this patient on medical therapy. We should at least try X, Y, and Z, before going to the cath lab.’”
Nonetheless, it remains a challenge to uptitrate medications in clinical practice, said Shah, calling it a “labor of love” for physicians. For that reason, if PCI can be safely performed to alleviate symptoms, particularly if the patient is young and still active, “I think that’s a very reasonable way to go,” he said.
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Rajkumar CA, Foley MJ, Ahmed-Jushuf F, et al. A placebo-controlled trial of percutaneous coronary intervention for stable angina. N Engl J Med 2023;Epub ahead of print.
Disclosures
- Al-Lamee reports speaker’s honorarium from Abbott Vascular, Fondazione Internazionale Menarini, Pharmaceuticals, Medtronic, and Servier Pharmaceuticals. She reports travel expenses from Philips and serving on an executive trial steering committee for Janssen Pharmaceuticals.
- Rajkumar reports speaker’s honorarium from Menarini Pharmaceuticals and consulting fees from Philips.





Comments