Positive Results With Carillon Mitral Device Seen in Real-world Registries
The device has evolved from one aimed at just functional MR to a broader HF therapy. EMPOWER will provide more insights.
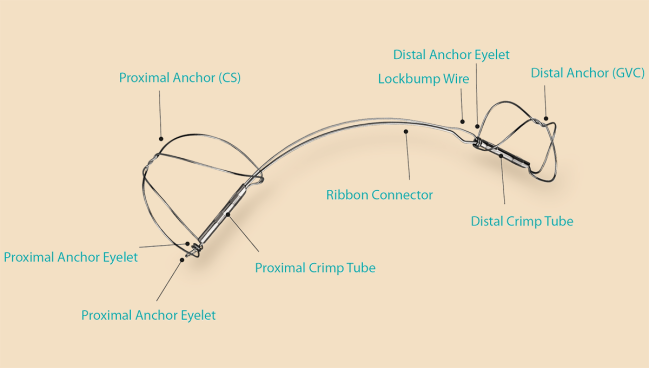
Photo Credit: Adapted from Cardiac Dimensions
BOSTON, MA—The Carillon mitral contour system (Cardiac Dimensions) not only treats functional mitral regurgitation (MR) but also appears to have a broader range of benefits for patients who have heart failure with reduced or preserved ejection fraction (HFrEF or HFpEF), according to real-world data out of Europe.
Among patients with up to 5 years of follow-up in the CINCH-FMR postmarket registry, use of the percutaneous device—which is anchored in the coronary sinus and cinches the mitral apparatus—safely reduced MR, improved NYHA class, and resulted in rates of clinical outcomes similar to what has been seen in earlier clinical trials, Klaus Witte, MD (University of Leeds and Leeds Teaching Hospitals NHS Trust, England), reported last week at THT 2025.
And as Holger Priebe-Brämer, MD (Marien Hospital Witten, Germany), reported during the meeting, in the largest single-center cohort currently available, the Carillon system reduced MR, vena contracta diameter, pulmonary artery pressure, NYHA class, and brain natriuretic peptide (BNP) levels within 30 days.
These results follow prior studies that have shown the intervention decreases left ventricular volumes in addition to reducing the severity of MR.
“We’re no longer closing our eyes to the fact that [for] the vast majority of patients with mitral regurgitation who have a dilated ventricle . . . there is no evidence of benefit of any other treatment in terms of device therapy at the moment,” Witte said. “So this is a serious patient need that we’re addressing here.”
The Carillon device has been around for many years, having received CE Mark approval in Europe in 2009.
“It’s a device that was initially intended as a mitral regurgitation therapy, but which has sort of since been retargeted as a heart failure therapy,” said Toby Rogers, MD, PhD (MedStar Heart & Vascular Institute, Washington, DC), who noted that the ongoing sham-controlled EMPOWER trial being conducted primarily in US centers is enrolling HF patients with any degree of functional MR.
The new studies presented at THT and others of the Carillon device, as well as promising data from other devices like the AccuCinch (Ancora Heart), all point in a favorable direction, Rogers told TCTMD.
“These type of devices that cinch the ventricle do seem to have benefits in patients with heart failure,” Rogers said. “They seem to have symptomatic benefits, with improvements in, for example, NYHA class. There seem to be benefits that you can measure with echo, such as reduction in the severity of functional mitral regurgitation. And there seem to be benefits in blood tests, such as BNP, that are a measure of congestive heart failure.”
Taken together, he added, that “is very encouraging for this entire field of geometric remodeling devices in heart failure.”
Commenting for TCTMD, Jeffrey Popma, MD (Cardiovascular Research Foundation, New York, NY), said, “What I find interesting about the Carillon device is that although it started out as a novel device to be placed into coronary sinus, reduce the area of the annulus, and reduce severe mitral regurgitation, it seems to have an added benefit of reducing ventricular volumes.”
That is what is being studied in the EMPOWER trial, which aims to enroll 300 patients with either ischemic or nonischemic cardiomyopathy, a dilated left ventricle, and symptomatic functional MR of mild or greater severity (≥ 1+). The primary endpoint, which will be evaluated with a win ratio analysis, encompasses all-cause mortality; heart transplant or left ventricular assist device; mitral valve surgery or percutaneous therapy; HF hospitalization; change in Kansas City Cardiomyopathy Questionnaire score; and change in 6-minute walk distance.
CINCH-FMR Postmarket Registry
The CINCH-FMR postmarket registry aims to enroll up to 500 patients with HF and functional MR treated with the Carillon system across multiple European centers. Witte presented data from an interim analysis of 228 patients (mean age 78 years; 51% women) who had up to 5 years of follow-up. They had high rates of comorbidities like hypertension (83%), atrial fibrillation (75%), and CAD (57%), and roughly half (49%) had an LVEF below 50%. Most patients (69%) had NYHA class III symptoms at baseline. MR grade was 2+ in 16%, 3+ in 57%, and 4+ in 27%.
The procedure improved MR by 1 to 3 months in 77.8% of patients (19.4% had no change and 2.8% had worsening). By 5 years, 85.7% of patients had a reduction in MR from baseline and 14.3% showed no change.
We’re not doing this to reduce their mitral regurgitation. We’re doing this to make them feel better with a long-term aim of reducing hospitalization and extending longevity. Klaus Witte
“But of course, none of this matters if our patients don’t feel better,” Witte said. “We’re not doing this to reduce their mitral regurgitation. We’re doing this to make them feel better with a long-term aim of reducing hospitalization and extending longevity. And again, the device does what we want it to do.”
At 1 to 3 months after the intervention, NYHA class was improved in 58.5%, maintained in 36.0%, and worsened in 4.0%. Corresponding figures at 5 years were 53.3%, 46.7%, and zero.
The survival rate was 53.1% at 5 years, similar to the 56.2% rate seen in a pooled analysis of prospective trials of the Carillon device. Rates of HF hospitalization and HF hospitalization/death were 52% and 67%, respectively.
Safety of the procedure, Witte said, was “overwhelmingly good.” The rate of device- or procedure-related events was 2.2% at 30 days and 2.6% at 1 year.
“The Carillon device . . . is clearly a device that is safe, provided you follow some simple rules,” Witte concluded. “It absolutely reduces mitral regurgitation in an unselected population of patients with both HFrEF and HFpEF. It improves patient symptoms and it’s associated with long-term clinical stability that align[s] well with the trial data from the open-label studies and the sham-controlled study, the REDUCE-FMR study that we published in 2019.”
A Single-Center Experience
Priebe-Brämer presented data on 201 patients (mean age 83 years; 68% women) treated with the Carillon device at his center between 2021 and 2024, after exclusion of three patients whose anatomy was not amenable to the procedure and who ultimately underwent mitral transcatheter edge-to-edge repair (M-TEER). These patients, too, had high rates of comorbidities, including atrial fibrillation (80%) and CAD (69%). Mean LVEF was 44%. Most patients (55%) had MR grade 2+ and the rest had MR grade 3+.
Safety was encouraging, Priebe-Brämer said, with no intervention-related deaths and three pericardial effusions after coronary sinus injury (1%). PCI was performed after compression of the left circumflex artery in 9% of cases, “but it was also no problem,” he said.
There was a learning curve for operators, with intervention time, radiation time, and contrast volume all declining over the study period.
You have a rapid learning curve because this intervention is quite easy. You get better in a few days. Holger Priebe-Brämer
Echocardiographic data showed that the mean diameter of the mitral annulus was reduced by 18% to 20% after 3 months. MR grade also improved, with two-thirds of patients achieving grade 1+ and one-third 2+ by that time point. Vena contracta diameter was reduced by 60% and mean estimated systolic pulmonary artery pressure by 20%.
Those changes were accompanied by an improvement in NYHA class. At baseline 96% of patients were in class III or IV. By 3 months, 98% were in class I or II. Over that span, BNP levels fell by 47% from a mean of 5,176 to 2,745 pg/mL.
Ultimately, 16% of patients required additional treatment for MR and underwent M-TEER.
“We have a high success rate, we have an excellent safety profile, because everyone was healthy, everyone was feeling better after that,” Priebe-Brämer concluded. “You have a rapid learning curve because this intervention is quite easy. You get better in a few days. You have large improvements in all items we measured.”
In addition, he said, “We have the ability to combine all alternative therapies we have with these interventions. And we have a preservation of all possible future therapies, like TEER and CRT.”
Awaiting EMPOWER
To TCTMD, Popma said the Carillon device is easy to implant into the coronary sinus, safe, and doesn’t preclude future treatment options like TEER or transcatheter mitral valve replacement (TMVR). Beyond treating MR, he added, “it seems that the Carillon device has fallen into the class of several innovative devices that are aimed at shrinking ventricle volumes.”
Addressing the inadvertent compression of the left circumflex artery with Carillon, Popma said better preprocedural CT screening can likely reduce this adverse outcome.
Overall, he said, “the big picture take-home is that, by reducing the commissural-to-commissural distance in the mitral annulus, the Carillon device has the opportunity to reduce mitral regurgitation and reduce ventricular volumes, which can be very useful in patients with dilated ventricles and mitral regurgitation.”
While the new Carillon data supports a focus on HF patients, “it’s still intriguing that they showed really quite impressive changes both soon after the procedure and durable changes in mitral regurgitation grade, for example, given that that’s really not even part of the current clinical evaluation in the US,” said Rogers.
The most interesting takeaway from these European studies on the Carillon device, Rogers said, is that “here is some data that suggests that, yes, it’s beneficial in heart failure, but it also has benefit on the mitral valve.”
He said the Carillon device has an advantage over some other devices, like the AccuCinch, because it’s a simple procedure, adding that there were no surprises regarding safety events. “I guess the only question is whether the complication rate in the real-world experience is any different to what they got in the clinical trials or what they will see in the clinical trial that is currently underway, but that’s not specific to this device,” he added.
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Witte K. CINCH registry: 5-year evidence of efficacy and durability. Presented at: THT 2025. February 12, 2025. Boston, MA.
Priebe-Brämer H. 200+ patients, real-world impact: Carillon therapy in routine care. Presented at: THT 2025. February 12, 2025. Boston, MA.
Disclosures
- Witte reports receiving consulting fees/honoraria/speaking fees from Abbott, Cardiac Dimensions, and Medtronic and grant support/research contracts from Medtronic.
- Priebe-Brämer reports speaking fees and direct financial support for educational meetings from Cardiac Dimensions.
- Popma reports that the Cardiovascular Research Foundation has “small ancillary responsibilities” related to the EMPOWER trial.





Comments