RADIANCE II Top-Line Results Promising for Ultrasound Renal Denervation
Three sham-controlled studies, including this US IDE trial, have been positive for the Paradise system. A fourth study flopped.
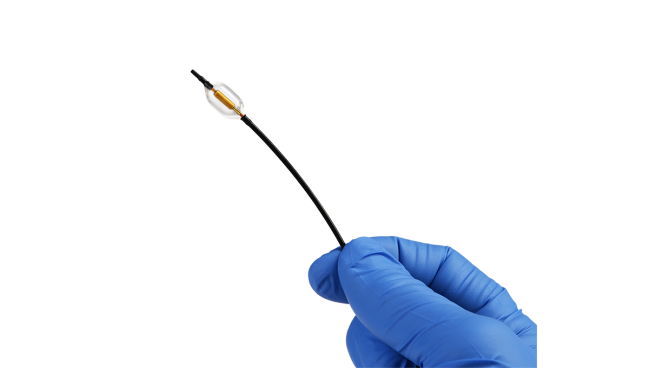
The ultrasound-based Paradise system (ReCor Medical) for renal denervation significantly reduces daytime ambulatory systolic blood pressure at 2 months compared with a sham procedure in patients with mild-to-moderate hypertension, according to preliminary data from a clinical trial required for US regulatory approval.
Top-line results of the RADIANCE II trial were released early Tuesday morning by ReCor and its parent company, but no information on the magnitude of the difference for that outcome, the primary efficacy endpoint, was included in the press statement.
The positive findings inch this technology closer to the US market: RADIANCE II, conducted at more than 60 centers across eight countries, including 25 US states, is the investigational device exemption trial required for the Paradise system to be cleared in the US. Investigators randomized 224 patients with mild-to-moderate uncontrolled hypertension who had been treated with up to two medications but who entered the study off antihypertensive therapy.
It is the third and largest positive sham-controlled trial within ReCor Medical’s global RADIANCE program, following RADIANCE-HTN SOLO in patients with mild-to-moderate hypertension and no background antihypertensive therapy and RADIANCE-HTN TRIO in patients with resistant hypertension on a background of triple antihypertensive therapy.
When ReCOR received approval for RADIANCE II, a company official said they planned to have four independently-powered, blinded, sham-controlled, randomized studies—SOLO, TRIO, RADIANCE II, and REQUIRE, for a total of nearly 500 patients—by the time they applied for premarket approval from the US Food and Drug Administration. REQUIRE, which was designed for regulatory approval in Japan and published in Hypertension Research earlier this year, enrolled patients in Japan and Korea with resistant hypertension and demonstrated no significant difference in BP reduction between renal denervation and the sham procedure.
Still, the RADIANCE II results add to a positive story that has otherwise been developing around renal denervation in recent years: the field has been rebuilding since the failure of SYMPLICITY HTN-3 put a damper on enthusiasm for the procedure.
The company plans to submit the accumulated results to the FDA as part of a premarket approval application for the Paradise system, which already has CE Mark approval in Europe. It has also launched the real-world Global Paradise System Registry, which will enroll up to 3,000 patients with uncontrolled hypertension.
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
ReCor Medical and Otsuka Medical Devices. ReCor Medical and Otsuka Medical Devices announce primary endpoint met in the RADIANCE II US pivotal trial of the Paradise system for the treatment of hypertension. Published and accessed on: July 26, 2022.




Comments