REBALANCE-HF Uncovers HFpEF Group That Responds to Splanchnic Nerve Ablation
The trial sets the stage for additional prospective studies of the approach before launching a pivotal trial, Marat Fudim says.
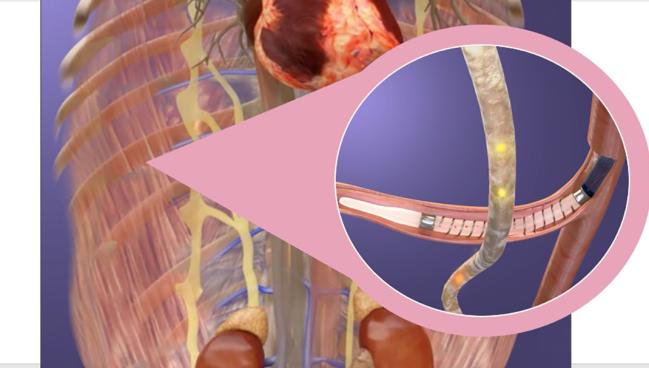
Photo Credit: Adapted from Levin H. AXON - percutaneous splanchnic nerve denervation. Presented at: THT 2022. New York, NY.
The sham-controlled portion of the REBALANCE-HF trial has demonstrated that ablation of the greater splanchnic nerve is a safe procedure and relatively easy to perform, with the potential to improve outcomes in the majority of patients who have heart failure with preserved ejection fraction (HFpEF).
In the overall trial population, the intervention—also called splanchnic ablation for volume management (SAVM)—did not have a major impact on hemodynamics at 1 month or clinical outcomes out to a year when compared with a sham procedure, Marat Fudim, MD (Duke University, Durham, NC), reported recently at the Heart Failure Society of America (HFSA) 2023 meeting.
Investigators did, however, identify a group of patients—making up about 55% of the cohort—who seemed to respond better to ablation, including those who had preserved cardiac output with exercise or standing, those who were able to augment their heart rate in response to exercise, those whose pulse pressure was maintained or improved when going from supine to standing, and those with less diastolic dysfunction.
These findings lay the groundwork for another prospective study that will aim to validate the benefits of splanchnic ablation in patients who are likely to be responders, before moving on to a pivotal trial of the technology, Fudim told TCTMD.
The REBALANCE-HF Trial
Splanchnic nerve ablation is designed to influence the distribution of blood in the body. The splanchnic compartment, which contains the liver, spleen, stomach, and intestines, contains about 30% to 40% of the total intravascular blood volume in the body and is highly innervated with autonomic nerve fibers, including the splanchnic nerves. Although a shift in blood from the splanchnic compartment to the heart and lungs is a normal physiological response to exercise, this can cause problems for patients with heart failure, including increased cardiac filling pressures, worsening symptoms, and a heightened risk of decompensation.
Ablation of the greater splanchnic nerve aims to head off this response to keep blood in the splanchnic compartment and ultimately improve outcomes in patients with HFpEF.
There is some promising preliminary evidence around the approach, including data from the REBALANCE-HF nonrandomized roll-in cohort that Fudim reported earlier this year suggesting that the procedure reduces pulmonary capillary wedge pressure (PCWP) and improves health status, functional capacity, and symptom burden through 6 months.
At the HFSA meeting, Fudim presented preliminary results of the randomized, sham-controlled portion of the trial, which was conducted at 18 centers. In total, investigators enrolled 26 roll-in patients to allow centers to gain experience with the procedure and 90 patients (median age about 72 years; 64% women) for the sham-controlled portion.
Inclusion criteria allowed for the enrollment of a broad range of patients with HFpEF. All had NYHA class II to ambulatory class IV symptoms, an LVEF of at least 50% over the past 3 months, ongoing stable guideline-directed medical therapy (GDMT) for heart failure and diuretics for more than 30 days, an exercise PCWP of 25 mm Hg or higher, and at least one of the following enrichment factors: at least one HF hospitalization in the past year, IV diuretics or intensification of oral diuresis for worsening HF in the past year, and an elevated NT-proBNP (with a relatively low threshold). A screening committee reviewed all patients before they underwent right heart catheterization with exercise before randomization.
Fudim called REBALANCE-HF a feasibility study with multiple objectives—to establish safety of the procedure, to ensure that it could be reliably reproduced across multiples sites and operators, and to identify responders for future studies.
Fudim said that splanchnic nerve ablation proved to be a relatively easy procedure to do, with interventional cardiologists, electrophysiologists, and heart failure physicians all performing it, with a median procedure time of 53 minutes. The nerve was successfully ablated in all but one patient in the treatment arm (98% treatment success).
And the procedure was safe, with no differences between the ablation and sham groups in device- or procedure-related serious adverse events through 1 month, or overall adverse events through 1 year. There was one death in the ablation arm related to a traumatic fall that was independently adjudicated to be unrelated to the procedure.
The primary efficacy endpoint was the change in PCWP 1 month after the procedure, with reductions during legs up and at 20W exercise of 2.0 and 1.1 mm Hg, respectively, in the treatment group and of 0.4 and 1.9 mm Hg, respectively, in the sham group among the overall trial cohort. The differences between the two trial arms were not significant.
On secondary endpoints, there were no significant differences between arms in terms of the degree of improvement in Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score, 6-minute walk test, or NT-proBNP, although the latter measure improved after ablation and worsened after the sham procedure.
Fudim noted that there were about twice as many add-ons of new medications or increases in doses, and 35 more unscheduled urgent visits involving a medication change, in the sham arm than in the treatment arm, which could have influenced the findings.
Identifying Responders
A key objective of the study was the responder analysis to identify those patients most likely to improve across a range of outcomes—including KCCQ score, 6-minute walk test, NT-proBNP, and PCWP—after ablation.
In this group—more than half of the trial population—there appeared to be a greater reduction in PCWP with legs up and 20W exercise (by 2.1 and 2.6 mm Hg, respectively) than seen in the overall cohort, although neither difference was significant compared with the sham group. There was, however, a significantly greater drop in work index PCWP (by 18 mm Hg/W/kg) and a greater increase in exercise duration (by 95 seconds; P = 0.02 for both) after ablation.
Improvements in KCCQ score, 6-minute walk test, and NT-proBNP at 6 and 12 months after ablation also seemed to be enhanced in the responder group, although only some of the changes were statistically significant compared with the sham group.
Overall, however, “we are confident . . . that the responder group is exactly the group that will see the most benefit,” Fudim said, adding that a confirmatory study that is either larger or focused on the responders will be necessarily before conducting a pivotal trial powered for hard clinical outcomes.
This intervention, he said, has the potential to provide another treatment option for patients with HFpEF. “We don’t have that many therapeutic options for these people with drugs and/or devices, so I think there is a lot of opportunity in this population.”
Discussing the results after Fudim’s presentation, Kavita Sharma, MD (Johns Hopkins Medicine, Baltimore, MD), pointed to several strengths of the study, including the sham control, the pragmatic inclusion criteria that allowed for the enrollment of multiple common HFpEF phenotypes, the high proportion of female participants, the high procedural success rate, the low rate of serious adverse events, and the identification of a responder subgroup.
But there were some limitations, too, including a discrepancy in the proportion of women in the two trial arms, low enrollment of underrepresented minorities, and the 11% rate of orthostasis among the patients treated with ablation. “While the majority were transient, this can be debilitating in HFpEF patients [and] may limit GDMT initiation, and who these patients are needs to be understood,” she said.
Sharma said there are several open questions about the procedure, including the need to better understand the patients who did not respond to ablation and were unable to augment cardiac output in response to exercise despite a preserved ejection fraction. “Is this related to preload insufficiency, RV failure, atrial myopathy, perhaps more restrictive physiology, chronotropic incompetence, or medications, or a combination of the above?”
Importantly, as noted by Fudim, she said, “a confirmatory randomized trial is likely needed with a potential focus on this responder subgroup.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Fudim M. Endovascular ablation of the right greater splanchnic nerve in HFpEF: primary analysis of the REBALANCE-HF randomized trial. Presented at: HFSA 2023. October 8, 2023. Cleveland, OH.
Disclosures
- The study was supported by Axon Therapies.
- Fudim reports financial relationships with Axon Therapies.
- Sharma reports support from an American Heart Association Go Red for Women SFRN Soter grant, an FNIH HeartShare grant, and Amgen. She has consulted or served on advisory boards for Amgen, Alleviant, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Cytokinetics, Edwards Lifesciences, Janssen, Novartis, Novo Nordisk, and Rivus Pharmaceuticals.




Comments