Solid Durability Seen With Surgical Resilia Tissue Aortic Valve
The 8-year data may hold value for TAVI—the newest Sapien valve uses the same tissue—but many other variables differ.
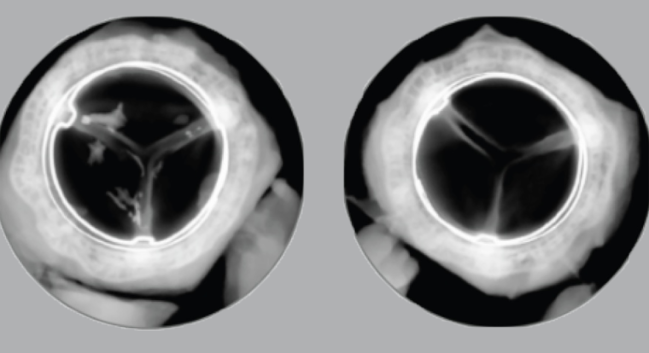
Calcium with control valve (left) and Resilia-tissue valve (right). Photo Credit: Edwards Lifesciences
Surgical bioprosthetic aortic valves made with bioengineered Resilia tissue (Edwards Lifesciences) appear to suffer less structural deterioration over 8 years compared with similar devices made of traditional bovine pericardial tissue, according to new data.
The findings represent the longest follow-up with the technology, which is designed to result in less calcification over time, and could have implications for TAVI given that the latest-generation Sapien 3 Ultra Resilia (Edwards Lifesciences) uses the same tissue.
“We talk about lifetime management, but if the first valve lasts longer, that is better than any reintervention,” said Tsuyoshi Kaneko, MD (Washington University School of Medicine in St. Louis, MO), who presented the study last week at the 2025 Heart Valve Society Annual Meeting.
“This data suggests that Resilia tissue may be that valve,” he told TCTMD. “We have to see the long-term [follow-up through] 10 years, 12 years—we’ve seen valves fail all of a sudden. So, we have to be cautiously optimistic, but at the same time, I think there is hope that this will be one of the best strategies for the lifetime management in younger patients.”
Danny Dvir, MD (Shaare Zedek Medical Center, Jerusalem, Israel), who nearly a decade ago raised the specter of early valve deterioration with some of the older-generation TAVI devices, agreed looking at the longer horizon on surgical valves helps out the interventional field. Though younger patients with aortic stenosis typically are treated with mechanical rather than bioprosthetic SAVR for their initial operation, “this presentation gives a lot of support to decreasing the age of patients having TAVI procedures, if we’re able to utilize the technology in second valves,” he said. “Our hope is that the Sapien technology and the second valve will be more durable over the years.”
Less SVD, Fewer Reoperations
The analysis included 689 patients from the COMMENCE study and 258 patients from the MAGNA EASE postapproval study who underwent SAVR with the Magna Ease + Resilia (Inspiris) and Magna Ease + TFX bioprosthetic valves, respectively. More than half of each cohort underwent isolated AVR surgery, with just under 20% receiving concomitant CABG and almost a quarter also having another procedure.
Notably, Kaneko said, the only difference between the two devices is the type of tissue used on the valve leaflets: Resilia or bovine pericardial tissue. That enabled a “compelling” comparison of the two arms to see the effects of the tissue itself without other complicating factors, he explained.
After propensity-score adjustment, mean patient age was 67.4 years, about one-third were female, and more than one-quarter were classified as NYHA III/IV at baseline.
Over 8 years, there were no differences in freedom from all-cause or valve-related mortality between the study arms. However, rates of freedom from major bleeding (90.4% vs 85.3%; P = 0.017), structural valve deterioration (99.3% vs 90.5%; P < 0.0001), reoperation (97% vs 90.5%; P = 0.001), and reoperation due to structural valve deterioration (99.2% vs 93.9%; P = 0.0007) were all higher with the Resilia valves.
Further, valves with Resilia tissue were associated with clinically stable hemodynamics throughout the 8 years, Kaneko reported.
The researchers will continue to follow the study patients out to 10 years, but Kaneko said he’s already convinced that SAVR valves with Resilia tissue should be chosen over those without.
Differences With TAVI
Annapoorna Kini, MD (Icahn School of Medicine at Mount Sinai, New York, NY), who was not involved in the study, called the data “very encouraging” but was reluctant to transpose the apparent success of the Resilia tissue seen here in SAVR onto the TAVI field.
“Given that TAVR is mounted on a flexible stent, unlike Inspiris with Resilia tissue mounted on a rigid surgical valve frame, we will need to determine the long-term outcomes of Resilia in a transcatheter aortic valve environment,” she said in an email. “Other factors, like having native aortic valve leaflets left in situ (vs in SAVR [where] native leaflets are resected), lack of commissural alignment, and frame underexpansion, may impact on Resilia tissue performance in TAVR mid- and long-term. Annual surveillance would be necessary to follow up these patients properly.”
Another of the study’s limitations is how it defines structural valve deterioration by relying on the 2008 definition and not more modern VARC-3 criteria, Kini said. “The authors should apply the contemporary definition to determine if their findings would be different,” she suggested.
We talk about lifetime management, but if the first valve lasts longer, that is better than any reintervention. Tsuyoshi Kaneko
Kaneko acknowledged this constraint, noting that the VARC-3 criteria weren’t available when the initial studies were conducted. “[We] don’t know whether these failures were all calcifications,” he explained. “We did dig a little more into the cause and we did see calcification on both sides, but the majority on the . . . old valve was stenosis, so likely a calcification, but [there’s] no way to prove it.”
Dvir agreed the definition used for structural valve deterioration is outdated. On the other hand, “the fact that we see less reoperation is a strong argument to suggest that the new technology has some merit and there is an advantage of using this,” he said.
While it would be better to have a randomized trial directly comparing valves with and without Resilia tissue, Dvir said what was presented “is good news for the community because people eventually don’t want mechanical valves. We need to have solutions to enable us to implant these devices without having structural degeneration.”
He’d eventually like to see the same type of comparison of Resilia in TAVI valves, as these will have their own set of behaviors that could affect structural valve deterioration. For now, “it’s a good signal to suggest that the mechanism of calcification is improving with this tissue treatment,” he commented.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Kaneko T. Propensity-matched 8-year outcomes following aortic valve replacement with novel versus contemporary tissue bioprostheses. Presented at: HVS 2025. Cairo, Egypt. April 18, 2025.
Disclosures
- Kaneko reports serving on advisory boards for Edwards Lifesciences, Abbott Vascular, and Johnson & Johnson and consulting for Medtronic.
- Dvir reports serving as a consultant to Medtronic, Abbott, and Edwards Lifesciences.
- Kini reports no relevant conflicts of interest.





Comments