Subclinical Leaflet Thrombosis After TAVI Linked to Higher Mortality
The study is at odds with others suggesting that HALT on CT typically resolves without causing later problems.
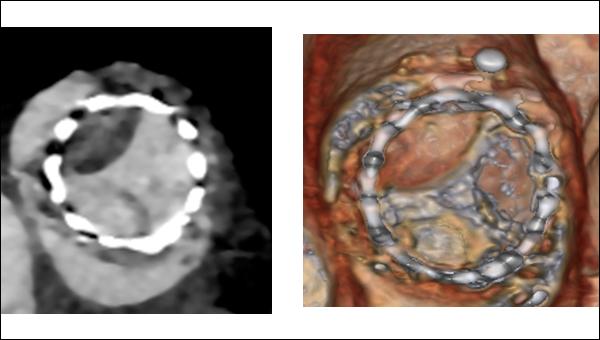
2D and volume-rendered images in short axis showing HALT with restricted motion in a SAPIEN 3 valve (Photo Credit: Santiago Garcia)
New observational evidence from a single US center suggests that subclinical leaflet thrombosis after transcatheter aortic valve implantation, even among patients treated with oral anticoagulation, is not a benign finding.
Among patients systematically screened with CT angiography 30 days after TAVI, patients who had signs of hypoattenuated leaflet thickening (HALT) were at a significantly increased risk of death during a median follow-up of 2.2 years compared with HALT-negative patients. Rates of stroke, transient ischemic attack, and heart failure hospitalizations did not differ between patients with and without evidence of HALT.
“We’re seeing a mortality signal here now for patients with HALT,” lead investigator Santiago Garcia, MD (Minneapolis Heart Institute Foundation at Abbott Northwestern Hospital, MN), told TCTMD. “It’s obviously unclear if this is related to confounders, if it’s related to HALT, or if it’s related to the treatment. You could make the case that warfarin is responsible and that we shouldn’t be treating this imaging finding. But it’s certainly the first study showing that HALT is not a benign entity.”
The new data, which were published online March 3, 2022, in Circulation: Cardiovascular Interventions, have raised questions about the potential risk of adverse clinical events in patients with HALT after TAVI. However, not everybody is certain the association with mortality is clear-cut, particularly since other recent studies have not documented a link between HALT and worse outcomes.
“I think we have to be very careful in interpreting this study and its findings,” Ole De Backer, MD, PhD (Rigshospitalet/Copenhagen University Hospital, Denmark), who wasn’t involved in the analysis, told TCTMD. In addition to pointing out that this is an observational study from one center, De Backer emphasized that HALT, or subclinical leaflet thrombosis, is a dynamic process and that many cases resolve spontaneously, making it difficult to draw a link all-cause mortality and a CT finding captured at 30 days.
I don’t think it’s something we should ignore—it’s something we need to understand. Santiago Garcia
“In most other observational studies so far describing leaflet thrombosis, there’s been no reported difference in clinical outcomes in patients with HALT and without HALT,” he said. “We also can’t forget those were pure observational studies—there was no intervention taken, no action on it. The physicians didn’t start oral anticoagulation. That’s the data we have so far, and that’s why there is the perception that it’s a benign finding. I still think that’s the true conclusion.”
In fact, De Backer is inclined to suspect that the higher risk of mortality might be linked to warfarin use.
“The mean age of the population here was older than 80 years old,” he said. “It’s an older, frail population, and there are numerous studies showing that starting anticoagulation in an elderly, frail population comes with a price, even with a price of higher mortality. That’s how I interpret this study: that oral anticoagulation might be the inducer for the higher mortality.”
What to Do About HALT?
The clinical implications of subclinical valve thrombosis, including reduced leaflet motion (RLM), have been hashed over since it was first identified in 2015. Back then, researchers published data from the PORTICO-IDE trial, as well as a pooled cohort of patients from two single-center registries, that showed RLM was seen in patients treated with multiple bioprothesis types, including transcatheter and surgical heart valves.
Subsequent registry studies showed that HALT and RLM was relatively common, occurring in roughly 10% to 20% of patients who underwent TAVI or surgical aortic valve replacement. As a result, the US Food and Drug Administration mandated CT imaging substudies be part of PARTNER 3 and the Evolut Low Risk Trial to better understand the natural history of untreated HALT. In both substudies, HALT was dynamic phenomenon that occurred more frequently after TAVI than after SAVR, but it was not associated with adverse cardiovascular events or impaired valve hemodynamics at 1 year.
At the Minneapolis Heart Institute, physicians began a screening protocol in 2015 following the first reports of subclinical valve thrombosis. After TAVI, patients underwent CT angiography at 30 days and those who showed signs of HALT, irrespective of the aortic gradient or symptoms, were started on warfarin for 3 to 6 months. Garcia said the decision to start oral coagulation can be debated, but they believed it was the right approach given the potential for stroke or TIA.
In total, 638 patients underwent TAVI between 2015 and 2019. The incidence of HALT at 30 days was 12%, with no difference between self-expanding and balloon-expandable valves (10% vs 14%; P = 0.16). Patients with HALT were older than those without it (median 83 vs 81 years; P = 0.002), were less likely to have diabetes, and were less likely to be treated with anticoagulants prior to TAVI. Mean aortic valve gradients at baseline and 30 days did not differ between HALT-positive and HALT-negative patients.
At 2.2 years follow-up, 24 of the 79 patients (30%) with HALT had died compared with 112 of 558 of patients (20%) without HALT (P = 0.02). In those with HALT, 41% of the deaths were attributed to cardiovascular causes, compared with 24% of deaths in the HALT-negative patients.
One of the strengths of the analysis is the number of HALT-positive patients, said Garcia, noting there were more cases than in PARTNER 3 and Evolut Low Risk Trial combined. Additionally, the observational analysis includes longer follow-up than those CT substudies.
“We have gone full circle, where the early signal was concerning, then we were reassured by the low-risk trials, but now again we’re seeing a signal [of harm],” said Garcia. “My take is that most people would agree that having a clot on the leaflet is not a good thing. Whether it’s linked to stroke, TIA, increased gradients, or even increased mortality, that remains to be seen. The concern that we all have is that it could also affect durability and there are some animal studies showing the link between calcification and degeneration. I don’t think it’s something we should ignore—it’s something we need to understand.”
Questions Linger
CT findings from the GALILEO-4D substudy, which was led by De Backer, did show that rivaroxaban (Xarelto; Bayer/Janssen) after TAVI was effective for reducing subclinical leaflet-motion abnormalities, but the overall trial was stopped early after it was shown rivaroxaban increased the risk of death and thromboembolic events. The ATLANTIS trial with apixaban (Eliquis; Bristol-Myers Squibb) didn’t demonstrate any harm with oral anticoagulation, but there was no benefit either. The CT substudy also showed that apixaban had no effect on rates of subclinical valve thrombosis compared with usual care.
I think we have to be very careful in interpreting this study and its findings. Ole De Backer
Both Garcia and De Backer agree the full story regarding the implications of HALT is not yet known and more studies are needed. Garcia said an ideal study would be to be to randomize HALT-positive patients to oral anticoagulation or standard care and then follow them for a number of years.
“That way you can really tell what’s the natural history of untreated HALT and what treatment can do,” said Garcia. “It would require a very large cohort, because this is a finding that affects roughly 10% of patients. The question then is where do you draw the line—where do you capture the patients who might have HALT? We did it at 30 days because of the signal [of harm] that came out of the PORTICO IDE study. We felt that if we were going to treat these patients, we better treat them early.”
De Backer noted that the effect of HALT on long-term valve durability remains an important question to address, particularly since TAVI is now being performed in younger, healthier patients.
“As we treat younger and younger patients, we want to give them the absolute best treatment that we can,” he said. “Maybe there, in that patient population, it might be justified to screen and treat the leaflet thrombosis if it’s present. This might not come with a penalty of higher mortality because the patients won’t have the same risk profile as in the elderly, frail patients. We can’t just conclude that if we see leaflet thrombosis that we don’t have to treat it—that might not be the correct conclusion either.”
In an editorial, Johny Nicolas, MD, and George Dangas, MD, PhD (both Icahn School of Medicine at Mount Sinai, New York), agree further studies are warranted, noting, like De Backer, that oral anticoagulation may work best if tailored to the patient’s specific risk profile.
“In the meantime, follow-up visits for patients who have undergone TAVR should be focused on a comprehensive clinical assessment and evaluation of bioprosthetic valve function using a transthoracic echocardiogram,” they write. “The use of CT imaging should be reserved for patients with alarming findings on echocardiography, who should then be maintained on therapeutic anticoagulation if HALT-related valvular dysfunction is detected.”
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Garcia S, Fukui M, Dworak MW, et al. Clinical impact of hypoattenuating leaflet thickening after transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2022;15:e011480.
Nicolas J, Dangas G. Hypoattenuated leaflet thickening after transcatheter aortic valve replacement: additional data, yet still many unanswered questions. Circ Cardiovasc Interv. 2022;15:e011828.
Disclosures
- Garcia reports consulting for Medtronic, Edwards Lifesciences, and Abbott Vascular, and institutional research grants from Edwards Lifesciences, Abbott Vascular, Gore, and BSCI.
- De Backer reports institutional research grants and consulting fees from Abbott and Boston Scientific.
- Dangas reports institutional research grants from Abbott Laboratories, AstraZeneca, Bayer, Boston Scientific, and Daiichi-Sankyo and consulting fees from Abbott Vascular, Biosensors, Boston Scientific, and Sanofi Aventis.




Comments