Two More Pig-to-Human Cardiac Xenotransplants Help Answer Many Unknowns
The procedures at NYU Langone Health were performed in brain-dead recipients and lasted 72 hours.
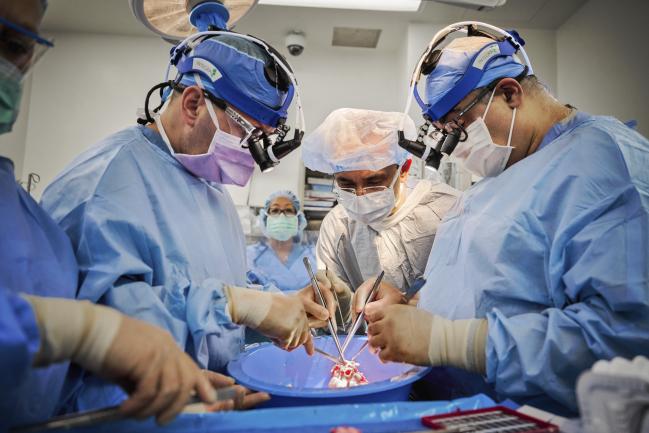
From left: Deane E. Smith, MD, Syed T. Hussain, MD, and Nader Moazami, MD, prepare the pig heart for xenotransplantation (Photo Credit: Joe Carrotta for NYU Langone Health).
Over the past 2 months, a team from NYU Langone Health in New York has successfully performed two cardiac xenotransplantation surgeries in brain-dead patients, the institution announced during a press briefing today.
For the procedures on June 16 and July 6, the medical team used the same genetically modified pig hearts manufactured by Revivicor (Blacksburg/United Therapeutics) that were used in the first pig-to-human heart transplant, performed in 57-year-old David Bennett at the University of Maryland in January. The recipients this time were 72-year-old Lawrence Kelly and a 64-year-old woman, whose name was withheld. Both had been declared formally brain dead—on ventilatory support and dialysis—and were not deemed eligible for traditional organ donation but seemed stable enough to undergo the transplant surgery for the purpose of gaining experimental insights.
“This is really a milestone and a stepping-stone in the right direction for someday hopefully making this clinically applicable to save the lives of many individuals,” said lead transplant surgeon Nader Moazami, MD (NYU Langone Health), during a press conference today.
Robert Montgomery, MD, PhD (NYU Langone Health), who last year successfully performed two pig-to-human kidney transplants, also in deceased recipients, is himself a heart transplant recipient. “It was just one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” he said in the press conference. “It is a great privilege for me to have witnessed that in my lifetime.”
Kelly’s wife, Alice Michael, said during the press conference that she was not originally aware of the opportunity for her husband to contribute to this kind of research. But because he had previously expressed the desire to be an organ donor, she said, “I didn't even have to think about the decision. I knew he would want to do it, and I had to do it. . . . It gives me comfort to know that he was in this research program and he can help so many people.”
Why Decedents?
The procedure was approved by a specially designated research oversight board at NYU Langone and the New York State Department of Health. Following the transplants, both patients were kept on ventilators and monitored for 72 hours.
Their decision to perform the procedures in decedents was multifactorial. “You have to think about what the intent is when you're performing an experimental procedure in a living human being; the focus is on keeping that individual alive, keeping them comfortable, and also getting them recovered and rehabilitated after the transplant,” Montgomery said. “For these studies that we're doing in the recently deceased, it's a completely different focus. The focus is really on learning and studying, measuring, and trying to really unravel what is going on in this brand-new incredible technology that is so complicated.”
This is really a milestone and a stepping-stone in the right direction for someday hopefully making this clinically applicable to save the lives of many individuals. Nader Moazami
Following both xenotransplants, the team took biopsies every day and performed invasive imaging as needed, according to Alex Reyentovich, MD, who serves as medical director of heart transplantation at NYU Langone Health. “We stopped at day 3 because that's where we felt was the best place to get the early information,” he said in the press conference. “Future studies will be longer.”
“We were able to, in these studies, really intensively look at tissue and blood samples [to have] a much deeper analysis of what's going on,” Montgomery said. In contrast, while it was a “tremendous feat” that the University of Maryland Medical Center team kept Bennett alive for 2 months, “in the end, we don't really know why that heart failed and why he died,” he added. “That is the limitation of doing a one-off transplant and not being able to intensively study it.”
The team believes the issue of hyperacute rejection has been solved through genetic modification of the pig hearts, but ongoing immunologic studies will continue.
“We hope to be able to profile exactly what happens at the molecular level, as well as the physiologic level in terms of what was the body's response,” Moazami said.
Lessons Learned
A big issue that has arisen in the xenotransplant field is the possibility of infection with porcine cytomegalovirus virus, which was found to be present in the heart given to Bennett following autopsy.
Montgomery confirmed to TCTMD that the screening process used for both of their procedures was improved and “more sensitive” than that used before Bennett’s procedure. “We also developed an in-house test for all these pathogens of concern,” he said. “Ensuring safety is a process that involves redundancy. It involves really scrutinizing your processes and your procedures.”
NYU Langone dedicated an operating room and purchased equipment specifically for these procedures that would not be used for other clinical purposes. “As we learn more, I think we'll realize whether we have overdone it or underdone it,” Moazami said. “I think the idea here is that by being cautious, eventually we can build a platform which can be more generally applicable so that we can do these operations in a clinical setting without necessarily taking all these precautions.”
Also, in primate studies and in Bennett’s case, it was thought that the porcine organs needed to be cooled and undergo perfusion in a special container in between being harvested and implanted in the donor. Here, Montgomery explained, they wanted to see if that process was essential or if the organs could be treated just as they would in a regular human-to-human transplant. Because of their success without using the special perfusion box to transport the hearts, he said, “we are beginning to wonder whether that pump is really a vital part of this. [It] would really limit the scalability of xenotransplantation. It would really limit the number of transplant centers that could participate in early xenotransplantation and the resources that would be involved—the cost of a xenotransplant.

“This is a great opportunity right now, in these decedents that we're studying to really determine what are the essential elements that are necessary for a good outcome when we do this in living humans,” Montgomery continued.
Another lesson the NYU Langone team learned was related to sizing. Due to the limited availability of pig hearts right now, the organ harvested for Kelly was found to be undersized, Moazami explained to TCTMD. “As a result of this, we had to make some surgical modifications to enlarge the blood vessels to be able to accommodate that mismatch,” he said. “Even though the heart function was absolutely fantastic throughout the 72 hours, there was some evidence that the amount of blood flow was not perfect enough.”
However, in the second procedure, the team was able to shave off about an hour of time in between harvest and implant, partially because the heart was properly sized but also because “we learned a lot of technical details from our first operation that prepared us,” Moazami added.
What’s Next?
Montgomery said he expects to see phase I clinical trials in cardiac xenotransplantation begin “sometime between now and 2025 . . . hopefully sooner rather than later.”
Despite calling their experience “a tremendous success,” Moazami stressed that their data are “still preliminary,” with many questions left to be answered before a phase I clinical trial can begin including the ideal patient recipients. “We're looking at these results with cautious optimism,” he said.
Reyentovich told TCTMD these procedures are part of an “an ongoing study” and they plan to extend the length of future studies beyond 3 days. “Generous donors don't come up that often,” he said. “When there's an appropriate donor, we're ready to do another one.”
Montgomery stressed the importance of awareness of this kind of “whole-body donation.” Interestingly, the family of the second xenotransplant recipient requested this option, “and that's the first time that that's happened,” he said. “It's really important that we continue to get that word out that there's now another way that we can give the gift of life at the time of your death.”
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
NYU Langone Health. Successful heart xenotransplant experiments at NYU Langone set protocol for pig-to-human organ transplants. Published on: July 12, 2022. Accessed on: July 12, 2022.


Comments