Ultrathin Struts Show Strength in Small Vessels: BIO-RESORT at 3 Years
Data from the RCT are bolstered by SCAAR registry findings that hint at a real-world ultrathin advantage.
PARIS, France—Patients with small vessels who receive ultrathin-strut stents are less likely to undergo repeat revascularization within 3 years than those who receive early-generation thin-strut devices, according to the latest round of results from the randomized BIO-RESORT trial.
“Thin[ner] stents may be particularly advantageous in small vessels due to the greater relative impact of strut size on lumen obstruction,” said Clemens von Birgelen, MD, PhD (Thoraxcentrum Twente, Enschede, the Netherlands), in a morning press briefing at EuroPCR 2019. Of the 3,514 patients enrolled in the three-arm trial, 1,506 had at least one lesion with a reference vessel < 2.5 mm.
von Birgelen presented the prespecified substudy of BIO-RESORT in a hotline session today. The results were simultaneously published online in JAMA Cardiology, with Rosaly A. Buiten, MD (Thoraxcentrum Twente), as lead author.
“There’s a pathophysiologic basis for it. It’s quite logical, and we feel that these findings may be clinically relevant,” von Birgelen said.
Also at EuroPCR, Swedish registry data—culled not just from small vessels but from a broader population—further hinted that ultrathin struts offer better outcomes, especially when it comes to TLR. The observational findings “corroborate the results of randomized clinical trials showing a potential incremental clinical benefit with ultrathin metallic DES use during PCI,” said study investigator Sergio Buccheri, MD (Uppsala University, Sweden).
BIO-RESORT and SCAAR Show Less TLR
BIO-RESORT enrolled patients at four Dutch centers between December 2012 and August 2015, randomizing them to receive the ultrathin sirolimus-eluting Orsiro (Biotronik), the very thin everolimus-eluting Synergy (Boston Scientific), or the thin zotarolinus-eluting Resolute Integrity (Medtronic), which have bare strut thicknesses of 60, 74, and 91 µm, respectively. In the small-vessel subset, 70% of patients treated were men and the mean age was 64 years.
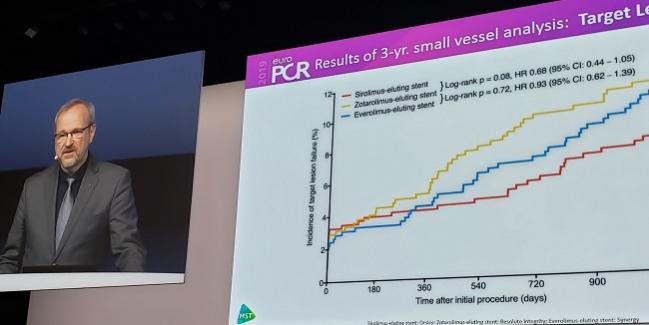
At 3 years, target lesion failure (defined as cardiac death, target-vessel MI, or TLR) on Kaplan-Meier analysis trended lower for the ultrathin versus thin groups (7.0% vs 10.0%; HR 0.68; 95%CI 0.44-1.05; P = 0.08). This nonsignificant difference was not seen when comparing the thin group with the very-thin group, which had a TLF rate of 9.5% (P = 0.72).
For TLR specifically, however, the ultrathin-strut devices outperformed the thin-strut stents by 3 years (2.1% vs 5.3%; HR 0.40; 95% CI 0.20-0.81; P = 0.009). That difference only emerged in years 2 and 3 (1.0% vs 3.7%; P = 0.006). The very-thin group had a 3-year TLR rate of 4.0% (P = 0.31 vs thin-strut stents).
Implantation of the ultrathin-strut stents independently predicted a lower risk of TLR by 3 years (adjusted HR 0.42; 95%CI 0.20-0.85). There were no disparities among the three devices when it came to cardiac death, target-vessel MI, or stent thrombosis.
In the same session, Buccheri presented results from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) on a cohort of nearly 75,000 PCI patients (mean age 67 years; approximately 74% men). For more than two-thirds, ACS was the indication for PCI. By 2 years, Orsiro had a definite stent thrombosis rate of 0.67%. Compared with a wide range of other next-generation stents, the ultrathin-strut device had a lower risk of TLR (1.6% vs 2.3%; P = 0.013) as well as a numerically lower rate of in-stent restenosis (1.5% vs 2.0%; P = 0.09). There was a slight though not significant increase in MI with Orsiro (6.0% vs 5.2%; P = 0.06).
How Much of It Comes Down to Struts?
In an editor’s note accompanying the JAMA Cardiology paper, Ajay Kirtane, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY), and Roxana Mehran (Icahn School of Medicine at Mount Sinai, New York, NY), observe that BIO-RESORT involves “three DES platforms that differ not only in the drug delivered and the polymer used to deliver it but also in stent strut thickness.”
Discussant Robert Byrne, MBBCh, PhD (Deutsches Herzzentrum München, Germany), said that the stents studied in BIO-RESORT are used day-to-day in cath labs. “This makes the data relevant for us,” he commented, though he called the cutoffs for thinness among the various devices “a little bit arbitrary” and noted that “there are other important differences between these stents” that mean the results merit caution.
Asked how sure he is that strut thinness lies at the root of the reduced TLR, von Birgelen told TCTMD that he is “quite confident.” Prior meta-analyses provide reason to rule out biodegradable polymers as the source of the benefit (of the three stents in BIO-RESORT, only Resolute Integrity has a durable polymer). Additionally, Orsiro’s biodegradable coating disappears over the course of 2 years, he said. “In Synergy, you have a minimal amount of coating that disappears within 4 months. So if there would be an advantage to having no coating as soon as possible, I would expect that the Synergy would have shown the better results. Apparently it didn’t matter that the Orsiro had this very slow process of degradation.”
Kirtane and Mehran, too, see a potential mechanism to support thinness. “Stent struts have been made progressively thinner over time as stent technology has iterated and thinner struts have been associated with greater flexibility (and deliverability) as well as improved clinical outcomes, which are thought to result from less abnormal coronary flow characteristics (rheology) after implantation,” they explain.
That the apparent differences didn’t emerge in the trial as a whole but rather in the small-vessel population “is relevant,” according to the editorialists, “because it supports the hypothesis that the comparative contribution of stent strut thickness may be greatest among vessels that are not adequately sized to tolerate thicker-stent struts with the greater degree of intimal hyperplasia that may result from these devices.”
Thinner may not always be better, they caution, citing lackluster results for chronic total occlusions from PRISON IV. However, the latest data “lend credence to a patient-specific and lesion-specific approach to device selection that is supported through the generation of randomized clinical evidence,” Kirtane and Mehran conclude.
After Buccheri’s late-breaking presentation of the SCAAR analysis, discussant Rasha Al-Lamee, MBBS (Imperial College London, England), said it’s valuable to see real-world data that back up RCT findings. She, too, referred to the biological plausibility. “It would make sense that if the struts are thinner, we would have less vessel injury [and] we would therefore have less inflammation, perhaps improved healing, and perhaps improvement in patient outcomes,” Al-Lamee commented.
But it’s always possible that confounders may be at play in an observational study, Al-Lamee noted. “I think we shouldn’t make too much from not statistically significant but numerical differences in terms of stent thrombosis or in-stent restenosis,” she stressed.
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Buiten RA, Ploumen EH, Zocca P, et al. Outcomes in patients treated with thin-strut, very thin-strut, or ultrathin-strut drug-eluting stents in small coronary vessels: a prespecified analysis of the randomized BIO-RESORT trial. JAMA Cardiol. 2019;Epub ahead of print.
Kirtane AJ, Mehran R. When can strut thickness really matter in percutaneous coronary intervention? JAMA Cardiol. 2019;Epub ahead of print.
Buccheri S. Real-life clinical outcomes with the use of an ultrathin sirolimus-eluting stent in Sweden: a report from the Swedish coronary angiography and angioplasty registry. Presented at: EuroPCR 2019. May 21, 2019. Paris, France.
Disclosures
- The BIO-RESORT subanalysis was performed without any external financial support. The BIO-RESORT trial was equally funded by Biotronik, Boston Scientific, and Medtronic.
- The SCAAR analysis was financially supported by Biotronik.
- Buiten and von Birgelen report receiving institutional research grants from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic during the conduct of the study.
- Kirtane reports receiving institutional grant support from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, CathWorks, Siemens, Philips, and ReCor Medical.
- Mehran reports receiving institutional grant support from AstraZeneca, BMS, Sanofi, Bayer, CSL Behring, DSI, BSC, OrbusNeich, and Abbott Vascular and consulting fees from Sanofi, Abbott Vascular, and Medscape.
- Buccheri reports no relevant conflicts of interest.


Comments