ACURATE IDE Trial Misses Primary Endpoint in All-Risk TAVI Patients
The result calls into question the prospects of FDA approval for the Acurate neo2 valve, which is already in use globally.

WASHINGTON, DC—The ACURATE investigational device exemption (IDE) trial has provided disappointing results, with the Acurate neo2 TAVI valve (Boston Scientific) falling short of noninferiority versus commercially available devices among patients with symptomatic severe aortic stenosis across the spectrum of operative risk.
At 1 year, the combined rate of all-cause mortality, stroke, or rehospitalization was 16.16% in patients treated with Acurate neo2 and 9.53% among those treated with valves from the Evolut (Medtronic) or Sapien (Edwards Lifesciences) platforms. The difference did not meet criteria for noninferiority with an 8% margin, Michael Reardon, MD (Houston Methodist DeBakey Heart & Vascular Center, TX), reported here at TCT 2024.
Reardon offered a range of theories as to why the trial did not deliver the hoped-for results, including the impact of the COVID pandemic on enrollment, staffing, supply chain problems, and operator experience. Sites saw, on average, nearly 3 months between Acurate neo2 implants, and most operators (72%) did five or fewer cases over the course of the study. Only 10% performed more than 10.
“That led to kind of an unfamiliarity with the valve,” Reardon told TCTMD.
A post-hoc review of cases found that underexpansion of the valve frame—something that could be corrected easily if identified during the procedure—occurred in about 20% of Acurate neo2 implants. “Valve underexpansion is something that the whole field is starting to recognize now as a real problem for long-term valve outcomes,” he said, noting that in analyses with only well-expanded valves, outcomes were much more similar between trial arms.
It remains to be seen whether these additional analyses will be enough to convince the US Food and Drug Administration that the Acurate neo2, which is already available in Europe and elsewhere, is safe and effective and should be approved.
“If you don’t get your primary endpoint, I suspect they won’t approve the valve a priori,” Reardon said. “My hope is that they’ll recognize that this is an identifiable problem that has a very reasonable, identifiable correction, now that we know about it, and then let us provide some further testing that shows them that if we do the things we need to do to assure full expansion, then we get results that are going to be equivalent to what the valves on the market [provide] today.”
Speaking with TCTMD, Jan-Malte Sinning, MD (Cellitinnen-Krankenhaus St. Vinzenz, Cologne, Germany), said that in his TAVI practice he uses about 50% Sapien valves, 40% Evolut valves, and 10% Acurate neo2 valves, adding that he was “a little bit surprised” by the findings of ACURATE IDE in light of the promising results from prior registries.
The main takeaway from the ACURATE IDE trial, Sinning said, is that “the overall performance of the Acurate neo2 is not bad. I mean, we have seen worse platforms. But at the end of the day, it’s not exactly the same standard of care that Evolut FX or Sapien 3 Ultra provides us with. . . . At least it seems like it’s not a valve platform for all-comers, and especially in calcified anatomies.”
For now, Sinning said, the Acurate neo2 is “an alternative—not less, but not more. . . . I wouldn’t say it’s a real competitor for transcatheter heart valves such as the Evolut FX and the Sapien 3.”
All Levels of Operative Risk
ACURATE IDE, conducted at 70 centers in the United States and Canada, is the first TAVI trial that includes all four levels of operative risk—low, intermediate, high, and extreme, Reardon noted. Investigators enrolled 1,500 patients (mean age about 79 years; 52% women) who had symptomatic severe aortic stenosis, were in NYHA class II or greater, had a documented aortic annulus size of 21 to 27 mm, and had an indication for TAVI.
They were randomized to receive the Acurate neo2 or commercially available balloon-expandable Sapien valves (Sapien 3 and Sapien 3 Ultra) or self-expanding Evolut valves (CoreValve Evolut R, Evolut PRO, Evolut PRO+, Evolut FX); about two-thirds received a Sapien valve and about one-third an Evolut valve.
Mean STS score was about 2.8%. Roughly a quarter (27%) of patients had high/extreme operative risk, 38% intermediate, and 35% low.
Procedural characteristics were largely similar in the Acurate neo2 and control arms, although the former had more frequent predilation during the index procedure (99.6% vs 33.0%) and postdilation balloon use (26.1% vs 11.3%; P < 0.001 for both). Predilation, with a recommended balloon size 1-mm smaller than the annular diameter, was required for the Acurate neo2.
The primary endpoint of the trial was the composite of all-cause mortality, stroke, or rehospitalization for valve-related symptoms or worsening congestive heart failure, assessed using a Bayesian analysis at 1 year. The posterior median difference between the Acurate neo2 and control arms was 6.63% (95% BCI 3.04%-10.20%), with the upper bound of the confidence interval exceeding the 8% noninferiority margin.
A Kaplan-Meier analysis showed a significantly higher 1-year rate of the primary endpoint (by 5.8%; 95% CI 2.4%-9.1%). Each component was at least numerically higher in the Acurate neo2 arm—death (5.0% vs 3.9%), stroke (5.7% vs 3.4%), and rehospitalization (5.3% vs 3.5%)—although only the difference in stroke was statistically significant.
Patients treated with Acurate neo2 versus commercial valves also had higher rates of CV death (3.7% vs 1.8%) and MI (2.4% vs 0.7%), with the latter finding driven by spontaneous events occurring more than 72 hours after the implant, “so it’s not something related to the procedure,” Reardon said.
Echocardiographic outcomes showed Acurate neo2 provided mean gradients similar to those seen with Evolut valves and lower than those seen with Sapien valves, as well as Doppler velocity index values that were comparable to self-expanding valves and better than the balloon-expandable valves.
At discharge, none/trace paravalvular regurgitation (PVR) was present in 75.9% of Acurate neo2-treated patients, 71.2% of those treated with Evolut valves, and 93.3% of those treated with Sapien valves, about what has been seen in other trials, Reardon said. Through 1 year, however, there was an unusual increase in mild PVR across all three groups. “This is not something we see in our trials. It’s not something I see in a pretty extensive clinical practice. So there’s some anomaly here that deserves a little more study, which we’re doing right now,” he said at a press conference.
The Underexpansion Issue
After seeing the results of the trial, the investigators dug into what might have gone wrong for Acurate neo2.
It did not appear to be related to difficulty in implanting the device. “The procedural outcomes for both arms were excellent. . . . The lack of experience did not affect the implant. And at 30 days, it matched control, and it only separated out between 30 days and 1 year,” Reardon said. In addition, echo parameters were as expected and there were no acute safety signals.
A case review identified underexpansion of the Acurate neo2 in roughly one-fifth of cases. Additional bench testing showed that fully expanded valves had low-velocity, laminar flow and adequate washout, whereas underexpanded valves had higher-velocity, turbulent flow, reduced washout, “and even a kink in the belly of the leaflet,” Reardon said. “To me, this is a very plausible reason for platelet activation, clumping, and microemboli. Those are the kind of things that can explain the myocardial infarctions after 72 hours and can be related to stroke.”
Further post-hoc analyses showed that underexpanded versus fully expanded valves had at least numerically higher 1-year rates of death, stroke, or hospitalization (18.8% vs 12.4%; P = 0.050), death (7.4% vs 3.7%; P = 0.054), and stroke (11.0% vs 3.5%; P < 0.001), and a nonsignificantly lower rate of rehospitalization (2.7% vs 5.9%; P = 0.131). When compared against the control valves, fully expanded Acurate neo2 valves had similar rates of adverse clinical outcomes through 1 year, “showing that if we really could expand these valves, we should really get a very good result,” Reardon said.
He said addressing underexpansion should be relatively straightforward, noting that operators didn’t know how to look for the issue when the procedures were being done and also often used smaller-than-recommended balloons for pre- and postdilation.
Underexpanded valves can be seen on imaging using the right anterior oblique view as the commissural posts not being parallel and the waist of the valve not being as wide as the valve height. If an operator sees that, “do a balloon and expand these valves. And if you do, then we would hopefully see results that look much more like the control results,” Reardon told TCTMD. “That’s an educational thing that I think we need to take forward as we create a checklist of things—here’s what you need to see.”
Unique Attributes
Reardon suggested that despite the missed primary endpoint in ACURATE IDE, the valve should not be abandoned, noting that it has some unique characteristics.
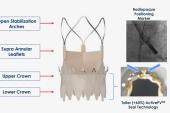
“One is it has a top-down deployment, which stabilizes [it] and allows you to accurately deploy a short-frame, self-expanding valve, not the long frame. And what this does is gives you some things that are like the balloon-expandable valve—rapid, reliable deployment, coronary access, and a low pacemaker [rate],” he explained to the media. “And yet it has the superior hemodynamics of a self-expanding valve and commissural alignment.”
Reardon told TCTMD “it would be a travesty to not have an opportunity to bring this valve to the US market because having used it, it’s a good valve. It has some potential real benefits.”
Sinning agreed that the Acurate neo2 has some positive attributes, including the combination of the features of a self-expanding valve, which is “less aggressive” than a balloon-expandable valve, and improved access to the coronary arteries. One caveat, however, is that “due to the lower radial forces, this platform might perform worse in heavily calcified annuli,” he added.
Also commenting for TCTMD, Poonam Velagapudi, MD (University of Nebraska Medical Center, Omaha), observed that while the ease of coronary access is a plus with Accurate neo2, currently available valves “have a very high standard and to actually get there and displace them, the new valve has to be equal or even better. . . . I don’t think we can say that this valve . . . is better than what we already have.”
Velagapudi, a member of the American College of Cardiology’s interventional cardiology council, said that if the FDA was still persuaded to clear this device, operators might struggle to “figure out which patients are those that this valve may be useful in.”
She added: “I don’t think at this time, given these results, I would be using this valve routinely as opposed to the valves that we already have.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Reardon M. One-year outcomes of Acurate neo2 vs approved TAVR devices in all-risk patients with severe AS: the ACURATE IDE trial. Presented at: TCT 2024. October 30, 2024. Washington, DC.
Disclosures
- Reardon reports consulting fees/honoraria from Boston Scientific, Medtronic, Abbott, Gore Medical, Anteris, J-Valve.
- Sinning reports being a proctor for Medtronic and Boston Scientific; being a member of scientific advisory boards for Abbott, Abiomed, Boston Scientific, Boehringer Ingelheim, and Medtronic; and receiving speaker honoraria and/or travel expenses from Abbott, Abiomed, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Edwards Lifesciences, Medtronic, Novartis, Novo Nordisk, Pfizer, Shockwave Medical, and Zoll.
- Velagapudi reports receiving speaking fees from and serving on an advisory board for Medtronic.





Comments