AGENT IDE: DCB Performance for In-Stent Restenosis Lasts Out to 2 Years
The DCB looks good in select cases, but given its high price tag, exactly when to use it is still up for discussion, say experts.
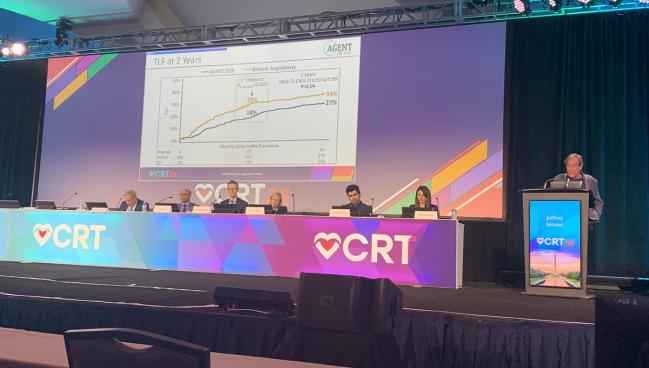
WASHINGTON, DC—A newly approved drug-coated balloon (DCB) for the treatment of in-stent restenosis (ISR) continues to be safe and effective, with fewer events and repeat interventions compared with balloon angioplasty, 2-year data show.
The Agent DCB (Boston Scientific) offered a lower rate of target lesion failure—the composite of ischemia-driven TLR, target vessel-related MI, or cardiac death—than did an uncoated balloon (27% vs 34%; P = 0.04). Follow-up is planned through 5 years.
“Consistent results were observed on the 2-year follow-up, with a significantly reduced instance of target lesion failure, with a 37% [relative risk] reduction,” Jeffrey W. Moses, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY), said here at CRT 2025. No cases of definite and probable thrombosis were seen in patients who received the DCB.
The device, which is coated with a low dose of paclitaxel, became the first coronary DCB in the United States to be approved for the treatment of ISR last year on the basis of early data from the AGENT IDE trial.
David Cohen, MD (St. Francis Hospital & Heart Center, Roslyn, NY, and Cardiovascular Research Foundation, New York, NY), told TCTMD the 2-year results are “reassuring, but perhaps not as durable as one might want to see in the real world.”
Similarly, session co-moderator Gregg Stone, MD (Icahn School of Medicine at Mount Sinai, New York, NY), noted that some “may quibble about whether or not there’s a little bit of a late catch-up” in the 2-year TLF rates, which had been 18% in the DCB group and 29% in the uncoated-balloon group at 1 year.
Importantly, he stressed that while these data pitted the DCB against plain old balloon angioplasty (POBA), the standard of care for ISR is DES.
Although that’s true, Moses said, the DCB is a welcome option for many operators who are hesitant to add another layer of stenting in a previously stented artery when confronted with multiple layers. “In smaller vessels it’s a matter of real estate,” he added. “As far as the first restenosis in a large vessel, we’ll have randomized data to help guide us in the future.”
Panelist Alaide Chieffo, MD (San Raffaele Scientific Institute, Milan, Italy), noted that the European Society of Cardiology (ESC) recently changed their recommendations, downgrading the evidence for DCB for in-stent restenosis and giving the first-line treatment recommendation to repeat DES. Long-term follow-up studies did not show a benefit of DCB over DES, which was the issue for the ESC task force given limited head-to-head data to go on.
“We need data at this point. Guidance and class recommendations come from randomized clinical trials,” she said.
AGENT IDE at 2 Years
AGENT IDE randomized 600 patients (mean age 68 years; 26% female; 7% Black) to treatment with the DCB or POBA. The cohort had high rates of diabetes (51%), multivessel CAD (79%), prior CABG (30%), and left main disease (22%). In 43% of patients, multiple prior stents were present at the site of the target lesion.
Patients were enrolled if they had ISR of a lesion previously treated with BMS or DES that was < 26 mm in length and between 2.0 and 4.0 mm in reference vessel diameter. Symptomatic patients were enrolled if they had target lesion stenosis < 100% but greater than 50%, and asymptomatic patients were enrolled if they had target lesion stenosis > 70%.
Conventional angioplasty techniques were used in all patients prior to randomization to the low-dose paclitaxel-coated DCB or uncoated balloon. Intravascular imaging was used in 72% of those in the DCB arm and 77% of those in the uncoated balloon arm.
Unfortunately, in the United States where this device costs about $5,000, it is a matter of economics. David Cohen
At 2 years, 86% of those in DCB arm were still on aspirin, as were 72% in the uncoated balloon arm, and 84% and 68% were still on dual antiplatelet therapy (DAPT), respectively.
TLR was less frequent in the DCB group (HR 0.56; 95% CI 0.40-0.80) out to 24 months, in line with the 1-year data.
Identifying the ‘Sweet Spot’
Stone noted that the DCB is pricier than a DES, raising issues of whether and how to control costs of these procedures while offering the most benefit to the patient.
Panelist James Hermiller, MD (Ascension St. Vincent Heart Center, Indianapolis, IN), said the “sweet spot” for Agent may be as a go-to option after a second stent, but he added that as a rule, it may not apply in every situation.
Cohen agreed that in the case of two stents, the DCB likely makes sense.
“But in the case of one [stent], it’s going to be dealer’s choice, and unfortunately in the United States where this device costs about $5,000, it is a matter of economics. In Europe, it’s not a big issue [because] it costs about $500,” he added. “So, it’s problematic for [US] operators when it’s so expensive and it’s not necessarily reimbursed.” Cohen noted that although the Centers for Medicare & Medicaid Services recently approved an additional reimbursement for the DCB, it still remains out of reach for many US patients.
Hermiller added that the reductions seen in repeat TLR in the AGENT IDE trial are pertinent to the economic discussion and should be studied further. “It’s relevant to the patient and it’s important to the hospital as well in terms of cost,” he said.
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Moses JW. Paclitaxel-coated balloon versus uncoated balloon for coronary in-stent restenosis: two-year outcomes of the AGENT IDE trial. Presented at: CRT 2025. March 8, 2025. Washington, DC.
Disclosures
- Moses reports equity interests/stock options in Covano, Orchestra Biomed, OSTIAL Corp, Weightless Lead, and Xenter.





Comments