Amulet LAA Closure Device Has Fewer Leaks Than Watchman: Does It Matter?
(UPDATED) The clinical relevance of the difference in peridevice leaks between devices remains unknown, experts say.
ORLANDO, FL—(UPDATED) The Amplatzer Amulet device (Abbott) seals off the left atrial appendage (LAA) better than the Watchman 2.5 or Watchman FLX devices (Boston Scientific) in patients with atrial fibrillation (AF) who are at risk for stroke, studies indicate, but it’s not clear that the difference in peridevice leaks has clinical consequences.
Compared with the first-generation Watchman 2.5, the Amulet resulted in fewer leaks ≥ 3 mm through 12 months, according to additional data from the AMULET IDE trial, which is consistent with the initial 45-day results, Dhanunjaya Lakkireddy, MD (Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, KS), reported here at TCT 2021.
And the smaller SWISS-APERO trial, presented by Roberto Galea, MD (Bern University Hospital, Switzerland), showed that even though there was no difference in residual LAA patency by cardiac CT angiography (CCTA) between the Amulet and the Watchman 2.5/FLX devices at 45 days, there were fewer transesophageal echocardiography (TEE)-measured leaks with the Amulet. The findings were published simultaneously online in Circulation.
Experts agreed that it’s better to have no leaks after LAA closure—the purpose of the procedure, after all, is to prevent thrombus in the appendage from entering the circulation and causing stroke—but whether the issue carries any relevance in terms of adverse outcomes down the line remains unknown. Of note, clinical outcomes did not differ based on device choice in either trial.
“Are these leaks clinically relevant or not? In my mind, every leak is an issue because you’re closing the appendage and you don’t want a leak,” Luigi Di Biase, MD, PhD (Albert Einstein College of Medicine at Montefiore Hospital, Bronx, NY), a member of the American College of Cardiology’s Electrophysiology Council, commented to TCTMD. “But the data about the clinical importance of these leaks still needs to be investigated in a big trial, and we don’t know what is the role of the leak at 45 days versus 12 months versus more than 12 months.”
AMULET IDE
The AMULET IDE trial randomized 1,878 patients with AF who were at high risk for stroke/systemic embolism to LAA occlusion using either the Amplatzer Amulet device or the Watchman 2.5. The primary results of the AMULET IDE trial, which supported US Food and Drug Administration approval of the Amulet device in August, show that all three primary endpoints assessing the completeness of the seal at 45 days, safety at 12 months, and effectiveness in terms of rates of ischemic stroke or systemic embolism at 18 months were met, with superiority of the Amulet device established for closure at 45 days.
The clinical importance of these leaks still needs to be investigated in a big trial. Luigi Di Biase
At TCT 2021, Lakkireddy reported on closure based on follow-up TEEs performed at 1 year. Between 45 days and 1 year, he noted, there was fluctuation of leaks, with improvement in some and worsening in others.
Even so, the superiority of the Amulet in terms of the percentage of patients with moderate peridevice leaks (≥ 3 mm) seen at 45 days (11% vs 26% with the Watchman 2.5) was maintained at 1 year (9% vs 22%; P < 0.001 for both).
Of patients who had moderate leaks at 45 days, those treated with the Amulet versus the Watchman 2.5 were less likely to still have leaks of that size at 1 year (39% vs 46%).
“The dual-seal Amplatzer Amulet left atrial appendage closure device demonstrated durably superior closure at 12 months compared to the single-occlusive mechanism Watchman 2.5 device,” Lakkireddy concluded during his presentation. The clinical impact of the leaks will be assessed over longer-term follow-up in the trial, which is set to check in on patients through 5 years, he noted.
SWISS-APERO
Galea said the SWISS-APERO trial, conducted at eight sites across four European countries, is the first to compare the Amulet device with the new-generation Watchman FLX in terms of residual LAA patency according CCTA at 45 days. The trial randomized 221 patients (mean age 77 years; 71% men) who had high bleeding risk, LAA morphology suitable for either device, and no LAA thrombus. In the Watchman arm, 22.7% of patients received the first-generation device and the rest received the Watchman FLX.
Major procedure-related complications were more frequent with the Amulet (9.0% vs 2.7%; P = 0.047), driven by a higher rate of BARC type 3-5 bleeding (7.2% vs 1.8%). There were two cases of device embolization, one in each group. Two patients died, both in the Amulet arm—one was related to an air embolism resulting in ischemic stroke and CV death and the other was due to a clinically relevant pericardial effusion treated by pericardiocentesis.
Galea noted that the complication rate was higher in SWISS-APERO than in previous studies of the Amulet device. In the AMULET IDE trial, for instance, the rate of procedure-related complications was 4.5% in the Amulet arm (versus 2.5% in the Watchman 2.5 arm). He pointed out, however, that the SWISS-APERO trial used a more-inclusive definition of procedural complications that incorporated related events that occurred beyond 7 days, as well as minor and nonclinically relevant events. In addition, patient characteristics indicated a higher-risk population in SWISS-APERO versus AMULET IDE.
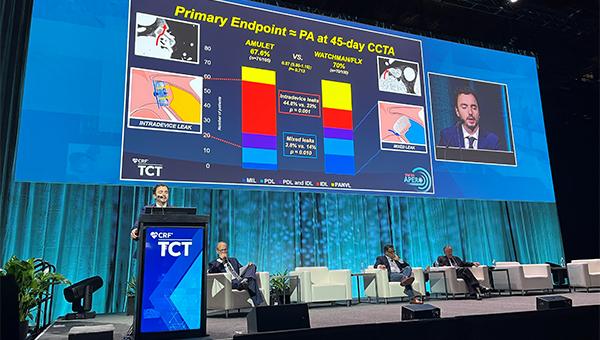
The primary endpoint was a composite of justified crossover to the other device during the procedure and CCTA-assessed residual LAA patency at 45 days. There was only one crossover—in a patient initially randomized to the Amulet device—so rates of the endpoint reflect residual LAA patency. There was no difference between the Amulet and Watchman groups (67.6% vs 70.0%; risk ratio 0.97; 95% CI 0.80-1.16). The finding was consistent across various subgroups and did not differ based on the generation of Watchman device used.
The distribution of the types of LAA patency differed between devices. The Amulet was associated with a higher proportion of intradevice leaks through the device lobe (44.8% vs 23.0%; P = 0.001), whereas the Watchman was linked to more mixed leaks, when movement of contrast is visible along part of the device lobe (14.0% vs 3.8%; P = 0.01), and patency with no visible leaks (21.0% vs 9.5%; P = 0.022).
We should try to investigate further to know how concerned we ought to be, and we want to make sure that it’s addressed prior to . . . just spreading this out more broadly to indiscriminate numbers of patients who we may or may not be helping. Ajay Kirtane
In terms of peridevice leaks on TEE at 45 days, a secondary endpoint, the rate was lower in the Amulet arm (13.7% vs 27.5%; P = 0.02). There were no leaks greater than 5 mm in either group, and only two patients with multiple leaks (both with Watchman 2.5).
Rates of device-related thrombus detected by CCTA or TEE at 45 days were low and did not differ between groups. There were no between-group differences in clinical outcomes at 45 days, although the trial was not powered for this purpose. The rate of a composite of CV death, stroke, or systemic embolism was 2.7% in the Amulet arm and 4.5% in the Watchman arm (P = 0.463).
“The clinical relevance of the residual LAA patency at 45 days still [remains] not entirely understood,” Galea said at a media briefing. He noted, however, that there is accumulating evidence from retrospective studies—including one published earlier this year—that suggests residual patency is associated with higher rates of thromboembolic events. “We cannot of course draw any conclusion [from] these retrospective studies, but I think that it’s still a target for . . . operators to complete the LAA sealing after the procedure.”
Aiming for Zero Leaks
In a discussion with reporters, physicians addressed the issue of leaks after LAA closure, even as the field awaits more-definitive data on the potential link to adverse clinical outcomes.
“We should try to investigate further to know how concerned we ought to be, and we want to make sure that it’s addressed prior to . . . just spreading this out more broadly to indiscriminate numbers of patients who we may or may not be helping,” Ajay Kirtane, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, NY), commented.
Ultimately, Ruby Satpathy, MD (Baptist Medical Center Jacksonville, FL), said, the goal should be no leaks, “and most of the time it is zero. . . . As we evolve this technology, as we’re getting into the new-generation devices, and also better preop understanding and intraop imaging, our goal is to go to zero because a lot of times we can tweak that appendage closure device, whichever you are choosing, just a little bit, recapture it and redeploy it, and actually have zero leak.”
In other words, she emphasized, “you can go from acceptable to optimal, and I think that responsibility lies in us.”
To TCTMD, Di Biase said the more-important point to take away when discussing differences between LAA closure devices is that it’s good for operators to have different options to consider. LAAs come in various shapes and sizes, so it’s helpful to be able to tailor the device to a specific patient. “The more devices we have, the more selection we have, the more we can suit them for our patients,” Di Biase said.
Thus, both the Amulet and the Watchman FLX device “have a very important role to play,” he said, noting he expects to see the procedural complication rate with the Amulet to come down over time, much like it did for the Watchman 2.5.
Speaking with reporters, Mintu Turakhia, MD (Stanford University School of Medicine and VA Palo Alto Health Care System, CA), underscored the importance of keeping an eye on these safety issues. And broadening the scope of the discussion beyond LAA occlusion devices, he said that “we don’t . . . have a proper randomized trial against the alternative therapy, which is a direct oral anticoagulant, for any of these. And when you look at these safety events and death, it’s something you need to consider if that would happen in patients on a direct oral anticoagulant because the eligibility [for LAA occlusion] nowadays is not a hard contraindication to oral anticoagulation.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Galea R, De Marco F, Meneveau N, et al. Amulet or Watchman device for percutaneous left atrial appendage closure: primary results of the SWISS-APERO randomized clinical trial. Circulation. 2021;Epub ahead of print.
Lakkireddy D. Amulet IDE trial: 12-month closure follow-up. Presented at: TCT 2021. Orlando, FL. November 5, 2021.
Disclosures
- The sponsor of SWISS-APERO, Insel Gruppe AG, Universitätsklinik für Kardiologie, was supported by local available funding and a research grant from Abbott.
- Galea reports no relevant conflicts of interest.
- The Amulet IDE trial was funded by Abbott.
- Lakkireddy reports consultant fees/honoraria/speakers bureau fees (personal) from Abbott Vascular, Biotronik, Janssen, and Johnson & Johnson.
- Di Biase reports being a consultant for Stereotaxis, Biosense Webster, Boston Scientific, Abbott Medical, and Rhythm Management; and having received speaker honoraria/travel support from Biotronik, Medtronic, AtriCure, Bristol-Myers Squibb, and Pfizer.
- Kirtane reports institutional funding to Columbia University and/or the Cardiovascular Research Foundation (CRF) from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Siemens, Philips, ReCor Medical, Neurotronic, Biotronik, and Chiesi. In addition to research grants, institutional funding includes fees paid to Columbia University and/or CRF for consulting and/or speaking engagements in which Kirtane controlled the content. Kirtane also reports consulting from IMDS and receiving travel expenses/meals from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron.
- Satpathy reports no relevant conflicts of interest.




Comments