BIOSTEMI at 2 Years: Orsiro Continues to Lower TLF in STEMI Patients
As event curves continue to diverge, the question of whether that’s due to the polymer or the struts may not matter.
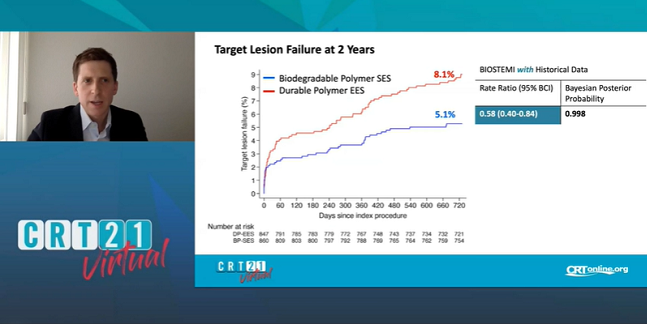
Between 1 and 2 years, the device-oriented advantage offered by an ultrathin-strut, biodegradable-polymer DES continues to accrue in patients treated for STEMI, the latest data from the BIOSTEMI trial show.
At 2 years, the rate of target lesion failure was 5.1% with the sirolimus-eluting Orsiro stent (Biotronik) and 8.1% with the thin-strut, durable-polymer, everolimus-eluting Xience Xpedition/Alpine (Abbott) stent, Thomas Pilgrim, MD (Bern University Hospital, Switzerland), reported this weekend during the virtual CRT 2021 meeting. At 1 year, the rates were 4% and 6% respectively.
“When we look at a landmark analysis with a time point at 1 year, we see actually that the difference still accrues over time,” senior investigator Juan F. Iglesias, MD (Geneva University Hospitals, Switzerland), told TCTMD. “The curves are still diverging,” which he said supports the idea that small differences in stent design can impact prognosis.
David Kandzari, MD (Piedmont Heart Institute, Atlanta, GA), a principal investigator for the pivotal BIOFLOW V trial (also with Orsiro), said that the findings speak to the fact that there is still room for improvement on top of the already excellent results seen with modern-day stents.
“Drug-eluting stents, certainly with the attention to structural heart disease and other novel therapies, have kind of taken a back seat in terms of the attention or focus of stent manufacturers,” he commented to TCTMD. “There’s a sense that it’s been less prioritized to iterate or develop new stent technologies. Similarly, for doctors there’s this misperception that all stents are equal now, that we’ve kind of plateaued.”
Data from various trials testing “the Orsiro stent remind us that differences can and do exist,” Kandzari stressed. “In a way, we need to reset our expectations with regard to when and where we might see differences between drug-eluting stents.”
Clinically Driven TLR
BIOSTEMI, an investigator-initiated trial, enrolled 1,300 acute STEMI patients at 10 centers in Switzerland; 94% had complete 2-year follow-up. As part of the study, the researchers performed Bayesian statistical methods incorporating data on 407 patients from the prior BIOSCIENCE trial.
The difference in target lesion failure, a composite of cardiac death, target-vessel reinfarction, and clinically indicated TLR, reached statistical significance—the posterior probability of superiority was 0.998, meaning that Orsiro was 99.8% likely to be superior. As hinted at by a trend seen at 1 year, the difference was primarily related to clinically driven TLR, but this time around it, too, significantly favored Orsiro (2.5% vs 5.1% with Xience Xpedition/Alpine; posterior probability of superiority = 0.993).
For target lesion failure, results were consistent regardless of whether stent diameter was ≤ 3.0 mm or ≤ 2.5 mm. They also remained significant when excluding the BIOSCIENCE cohort.
However, “despite a significant difference in device-oriented clinical outcomes, there was no significant difference in the composite patient-oriented clinical outcome [of death, MI, or any repeat revascularization] or individual safety endpoints such as death, myocardial infarction, and stent thrombosis,” Pilgrim et al note.
“Therefore,” said Iglesias, “we still need some improvements in care beyond the stent itself to improve the outcomes of our patients following ST-segment elevation myocardial infarction.”
The current analysis, which was published online in JACC: Cardiovascular Interventions, also suggests that Orsiro’s lead is augmented among patients with multivessel disease (Bayesian posterior probability = 0.994), a finding that has been confirmed in subsequent, as-yet-unpublished analyses, he noted. “There may be a benefit not only when we treat one lesion; this effect seems to accumulate with an increasing number of lesions treated.”
Polymer, Struts, or Both?
It’s tempting, Iglesias acknowledged, to try to pinpoint which stent component—the polymer or the strut thickness—is behind BIOSTEMI’s findings. Very likely, it’s the device as a whole that matters, he suggested.
Like Iglesias, Kandzari said he often encounters questions over whether it’s the struts or the polymer at work in Orsiro’s outcomes. “It’s ultimately speculative,” he said, but one clue is that both BIOFLOW V and BIOSTEMI found no effect on outcomes by stent diameter, meaningful because the larger stents had thicker struts. “At first glance, that would argue against it being strut thickness and [suggest] maybe instead it’s the polymer.”
On the other hand, the “totality of data” indicate thinner is better when it comes to struts and in BIOFLOW V there were early differences in outcomes, such as MI, even when the biodegradeable polymer was still present, Kandzari continued.
It may be that each of these design features has something to offer, he said.
In an editorial accompanying the BIOSTEMI paper, Robert A. Byrne, MB BCh, PhD (Mater Private Hospital, Dublin, Ireland), and J.J. Coughlan, MB BCh (Royal College of Surgeons in Ireland, Dublin), focus their energies on the polymer, asserting that it’s not yet established whether biodegradable or durable is best for the STEMI setting.
“Autopsy studies, derived predominantly from cases early in the experience with DES, suggest that inflammatory reactions to polymers may be of higher relative importance in the setting of STEMI compared with stable coronary artery disease,” they write. “High thrombus burden and penetration of stent struts into plaques with necrotic core are specific characteristics of intervention in this setting, which may affect vessel healing poststenting.”
The current results are welcome, Byrne and Coughlan say, given that “dedicated head-to-head data in this setting are sparse.” In their view, though, the 1-year advantage for Orsiro was merely “maintained at 2 years.” They conclude, “Expressed in Bayesian terms, it remains to be seen whether the present data are sufficient to update our a priori beliefs.”
BIOSTEMI had been set to end at 2-year follow-up but will now continue through 5 years, according to Iglesias. Paired with the BIOFLOW V trial, which excluded STEMI patients, “we have convincing data across the whole spectrum of coronary artery disease that this stent provides superior outcomes when compared to contemporary second-generation drug-eluting stents,” Iglesias observed.
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Pilgrim T, Muller O, Heg D, et al. Biodegradable- versus durable-polymer drug-eluting stents for STEMI: final 2-year outcomes of the BIOSTEMI trial. J Am Coll Cardiol Intv. 2021;14:639-648.
Byrne RA, Coughlan JJ. Biodegradable- versus durable-polymer DES in ST-segment elevation myocardial infarction: time to update our a priori beliefs? J Am Coll Cardiol Intv. 2021;14:649-652.
Disclosures
- BIOSTEMI trial was an investigator-initiated study supported by a dedicated research grant from Biotronik.
- Pilgrim has received research grants to the institution from Biotronik and Boston Scientific, has received speaker fees from Biotronik and Boston Scientific, and is a consultant for HighlifeSAS.
- Iglesias has received a research grant to the institution and personal fees from Biotronik during the conduct of the study, research grants to the institution and personal fees from Biotronik, Philips Volcano, Abbott Vascular, and AstraZeneca, and personal fees from Terumo, Medtronic, and Cardinal Health outside the submitted work.
- Byrne has received research funding to the institution of prior employment from Celonova Biosciences and has received research or educational funding to the institution of current employment from Abbott Vascular, Biosensors, Biotronik, and Boston Scientific. He has not received personal payments from any pharmaceutical company or device manufacturer.
- Coughlan reports no relevant conflicts of interest.
- Kandzari reports receiving institutional grant support from Biotronik, Abbott, and Medtronic as well as personal consulting honoraria from Medtronic.




Comments