CAST-HF: Could Myocardial Shockwave During CABG Yield Cardiac Regeneration?
A small, sham-controlled trial of intraoperative shockwave offers an “intriguing” hint that regeneration might be safe and feasible.
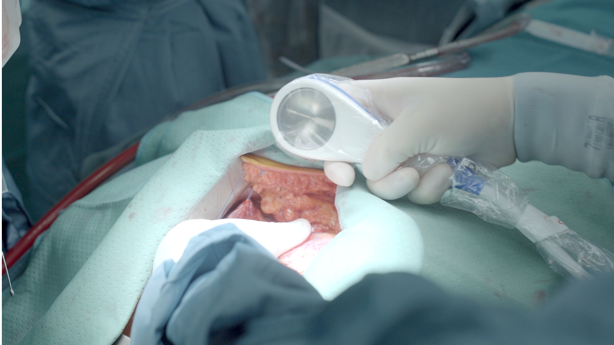
Photo Credit: Anita Kapferer, TirolTV
Intraoperative shockwave therapy as an adjunct to CABG surgery appears to improve left ventricular function over 1-year follow-up, while also leading to improvements in a range of patient-reported metrics, the small CAST-HF trial suggests.
Johannes Holfeld, MD (Medical University of Innsbruck, Austria), who presented the CAST-HF results at the European Association for Cardio-Thoracic Surgery (EACTS) 2022 meeting last weekend, predicted that shockwave, delivered directly to the surface of the heart during cardiopulmonary bypass, could give surgery an edge over PCI in stimulating myocardial recovery in heart failure.
That rosy forecast comes within months of REVIVED-BCIS2 showing that PCI—unlike surgery, in the 10-year results from the much earlier STICH trial—was no better than guideline-directed medical therapy in improving outcomes in patients with severely impaired LV function and extensive coronary artery disease.
“I truly believe that this technology could cause a paradigm shift in the treatment of ischemic cardiomyopathy,” Holfeld said. “For the first time, this unmet clinical need of myocardial regeneration could become a reality.”
Over the last several decades, the range of experimental therapies targeting the elusive goal of myocardial regeneration secondary to obstructive coronary disease have involved stem cells, gene therapy, cellular “reprogramming,” and tissue engineering but none, to date, have successfully demonstrated an effect on heart muscle healing.
This unmet clinical need of myocardial regeneration could become a reality. Johannes Holfeld
Holfeld invented this particular shockwave technology and is the founder, a shareholder, and CEO of the company making the shockwave device. In preclinical studies, he and his colleagues have previously shown that shockwave therapy helped reduce fibrotic scar tissue and induced angiogenesis in ischemic myocardium. According to the CAST-HF protocol paper, an unpublished pilot trial of 10 patients in 2008 showed no severe side effects of direct myocardial shockwave such as arrhythmias, hematoma formation, or lacerations.
As Holfeld stressed to TCTMD, shockwave in this setting is different from other applications, such as lithotripsy (used for breaking down kidney stones and severely calcified lesions in coronary, valvular, and peripheral artery disease). The sonic pressure waves applied to the heart following CABG surgery are at a specific energy level and frequency shown to release microvesicles from cells, which in turn stimulates angiogenesis and inflammatory processes required for tissue regeneration.
Direct to the Heart
For the current study, Holfeld et al randomized 63 consecutive patients with heart failure with reduced ejection fraction (HFrEF, defined as LVEF < 40%) undergoing CABG surgery either to interoperative shockwave therapy or to a sham procedure. Prior to surgery, more than two-thirds of patients in both groups were in NYHA class III to IV heart failure with mean baseline LVEF of approximately 31% in both groups.
In the active therapy group, the Cardiac Shockwave Probe (Heart Regeneration Technologies GmbH, Innsbruck, Austria) was applied directly to the myocardium with continuous saline rinsing to maximize coupling of the epicardium and the shockwave device. Shockwave application was 0.38 mJ/mm2 at a frequency of 3 Hz, applied directly to areas of ischemic myocardium over a 10- to 15-minute period.
In the sham group, the probe was placed against the exposed epicardium, but without shockwave delivery. To minimize bias, allocation to either active therapy or sham was done following the surgical procedure, with patients remaining blinded to randomization group and shockwave was delivered to the heart while all patients remained on cardiopulmonary bypass.
No procedure-related adverse events occurred, said Holfeld, adding to TCTMD that the procedure is painless for patients and there is no risk to anastomoses “if handled with care.”
This is pretty amazing to see. Patrick O. Myers
By 6 months, LVEF had improved from baseline by 5.39% in the sham group compared with 10.36% in the shockwave group (P = 0.009). By 1 year, those improvements were 6.61% and 11.96%, respectively (P = 0.022).
“This is an almost 12-percentage-point improvement of the ejection fraction after 1 year, which in my view is spectacular,” Holfeld said. “I want to remind you that stem cell trials have seen improvements of [just] 5 to 6%.”
Investigators also saw clear trends towards reductions in end-systolic and end-diastolic volumes, but these differences were underpowered, he noted.
Secondary endpoints, including 6-minute walk test and quality of life scores on the Minnesota Living With Heart Failure Questionnaire, improved in both study arms, but gains were significantly greater in the shockwave group. In fact, said Holfeld, at 12 months, “patients [in the shockwave group] were able to walk twice as far as the untreated patients did.”
“Cardiac shockwave therapy adjunctive to CABG surgery is safe and significantly improves left ventricular ejection fraction,” Holfeld concluded. “We see an improved physical capacity, an improved quality of life, and we can anticipate that this treatment will lead to a lower mortality.”
A Holy Grail
Speaking with TCTMD, EACTS council member Patrick O. Myers, MD (Lausanne University Hospital, Switzerland), cautioned that whether a 6% difference in ejection fraction “is going to make a huge difference in hard outcomes” remains to be seen. “Obviously what we would be interested in are studies with more patients, looking at hard outcomes, and longer follow-up.”
But the findings so far are intriguing, he continued. “I must say I didn't have this at all on my radar,” said Myers. “What I think is pretty striking is that we've . . . seen lots and lots of promising animal models of stem cell therapy, regenerative medicine, all kinds of things like that, but once you go to an actual patient, the results are really not that striking or not as good as was promised in the preliminary work. So this is pretty amazing to see.”
Also compelling, he noted, was how consistent the results are across the endpoints, with patient-reported changes going in the same direction as the LVEF, at a magnitude appropriate to the improvements seen on MRI.
The fact that shockwave already has an established track record not only for wound healing and soft tissue therapies, but also for vascular access in coronary and structural heart disease is appealing, he said, as is its simplicity as compared with, for example, stem cell therapies.
“This is, I believe, a very well conducted, prospective, randomized sham-controlled trial that really provides some interesting, hopeful results,” Myers said.
There is a history of numerous regenerative therapies demonstrating efficacy in small studies which failed to stand up when adequately powered, so I think we should be cautious at this stage. Tom Cahill
Also commenting on the findings for TCTMD, Tom Cahill, MBBS, DPhil (Oxford University, England), called myocardial regeneration a “holy grail” in cardiac research, “long pursued” yet remaining “tantalizingly out of reach. Over the years the cardiovascular community has had its fingers badly burned by trials of cell therapy for heart regeneration, which generated enormous hype but have ultimately failed to deliver.”
These results, he said in an email, “are intriguing and suggest there is mileage in further investigation of this device,” adding that it’s refreshing to see regenerative therapies being tested in blinded, randomized studies with use of sham control and a cardiac MRI-based endpoint.
“Personally, I maintain a healthy skepticism pending validation of these findings in larger-scale studies,” he said, noting that just 42 of 63 randomized patients completed 1-year follow-up with MRI. “There is a history of numerous regenerative therapies demonstrating efficacy in small studies which failed to stand up when adequately powered, so I think we should be cautious at this stage. In addition, from a biological perspective, it is unclear precisely what this therapy is doing—although multiple cellular effects have been identified in animal models, to have true confidence in a novel clinical therapy, I do think it is necessary to clearly understand the primary mechanism of action.”
Holfeld acknowledged the need for further studies. noting that a multicenter, European CAST-HF 2 trial is slated to launch before the end of the year. A US trial has also received Food and Drug Administration “breakthrough therapy” designation, and several US centers are already committed to participate in the trial, which Holfeld anticipates starting in the spring of 2023.
“I understand that for many, this is at an early stage, but I want to say that there are two aspects that clearly distinguish our technology from stem cell therapy,” said Holfeld. “One is we have been able to fully describe the molecular working mechanism—there are numerous papers from my research group that describe this mechanism, whereas with stem cells we still today do not really know how they work. That’s a big difference.
“The second differences is that we know the advantages of this therapy,” he continued, adding, “It’s been used in traumatology and orthopedics for many years, and we therefore have experience from millions of patients for years: we know that there are no long-term negative side effects like malignancies, and we know that the effect of shockwave is a stable long-term effect.”
Unlike gene- and cell-based approaches to regeneration, Holfeld added, shockwave does not introduce any foreign elements but rather invokes endogenous healing in a targeted manner. “We need these randomized controlled trials,” he told TCTMD, but “I am very optimistic.”
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Holfeld J. Cardiac Shockwave Therapy in patients with ischemic heart failure (CAST-HF): a prospective, randomized-controlled trial. Presented at EACTS 2022. Milan, Italy. October 8, 2022
Disclosures
- Holfeld is a shareholder, inventor, and CEO at Heart Regeneration Technologies GmbH.




Comments