Closing the Mitral Gap: Early Pascal Experience Shows Promise for High-Risk MR Patients
Transcatheter mitral valve repair was feasible in a small population deemed ineligible for MitraClip and resulted in good functional improvement.
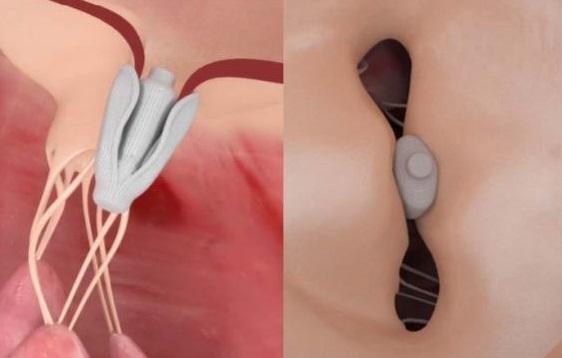
Potentially fulfilling an unmet need among patients with mitral regurgitation (MR) who cannot be treated with MitraClip, the Pascal transcatheter mitral valve repair device can feasibly be implanted and reduce regurgitation severity, according to a first-in-human study.
For patients deemed too high risk for surgery, MitraClip (Abbott) has been the only percutaneous treatment available since its approval by the US Food and Drug Administration in 2013. But “a considerable proportion of patients” are not eligible to receive MitraClip for anatomical reasons, according to lead study author Fabien Praz, MD (University Hospital Bern, Switzerland), and colleagues.
Designed on the same edge-to-edge repair principle as MitraClip, the Pascal system (Edwards Lifesciences) is slightly larger and wider, features longer paddles and a central spacer, and allows for independent leaflet grasping—all features that make it more feasible to use in patients with complex anatomy, Praz noted to TCTMD.
Examining Pascal
For their study, Praz and colleagues looked at 23 patients with moderate-to-severe or severe MR who underwent implantation with the Pascal device between September 2016 and March 2017 under compassionate use criteria in five countries. All but one of the patients were classified as NYHA class III or IV, and the cause of mitral regurgitation was functional in 12 and either degenerative or due to mixed mitral valve disease in the remaining 11.
Implantation of at least one device was successful in all patients, with a resulting procedural residual MR of 0+ or 1+ in 74% and 2+ or less in 96%. Six patients received a second device for either MR reduction optimization or correction of intraprocedural partial detachment of the first device.
Taking into account the partial leaflet detachment in one patient, technical success (the primary endpoint) was achieved in 96% of patients, and periprocedural complications were minimal—one TIA and one minor bleed at the access site. One patient, who had residual MR grade 3+ postimplantation of one device, died after the procedure of refractory heart failure. The remaining patients were discharged home within a mean of 4 days.
At 30 days, three patients had died from cardiovascular causes and device success was achieved in 78%. Except for one patient with an elevated transmitral gradient, all patients had functional improvement by the end of the study, with 95% classified as NYHA class I or II.
Praz told TCTMD that he was slightly surprised by the “high degree of efficacy” seen with the device. “In this population with difficult anatomy, we were actually able to really have a good reduction of mitral regurgitation,” he said. “Seventy-seven percent of the patients had a grade zero to one reduction, which is really a good result for a first-generation device.”
Still, these are “very early” data, Praz emphasized. The ongoing CLASP study, in which he is involved, is designed to enroll 130 patients and follow them through 3 years. “We'll certainly need 1 year and even longer to look at how the remodeling can take place and how patients are improving after the implantation of this device,” he said.
The ultimate goal with the Pascal system is to “improve the efficacy of transcatheter mitral valve repair, to extend the suitable population, and to facilitate the intervention,” Praz concluded.
Familiar, Yet Innovative
In an editorial accompanying the study, Ottavio Alfieri, MD and Nicola Buzzatti, MD (San Raffaele Scientific Institute, Milan, Italy), write, “The invention of a new device that targets the mitral valve percutaneously represents a potentially important contribution to the treatment of mitral regurgitation, and deserves special attention from the cardiology community.”
Everybody will be very keen to see where this fits. Paul Sorajja
Indeed, “any therapy—investigational or otherwise—that is expanding the potential population of patients with MR who can be treated is welcomed and very important,” Paul Sorajja, MD (Minneapolis Heart Institute, MN), who was not involved with the study, told TCTMD. “This study, which focused on patients who apparently were not eligible for current therapies, is very innovative and provocative in that sense because we're always looking to expand the boundaries of what we do.”
However, he said further scrutiny is needed to determine if a patient truly cannot be treated with MitraClip. In this study, “there is virtually no description what pathology they mean by being ‘ineligible for MitraClip,’” Sorajja noted. Current experience with MitraClip has shown that the “therapy can treat almost any patient,” he said. “So to have a patient population who is truly ineligible for MitraClip, I think that needs further description.”
Still, the Pascal system “adds innovation that has not been made for catheter-based leaflet repair in a number of years,” Sorajja said. “It’s a welcome addition, because the transeptal/transfemoral approach that this technology employs is very familiar to many . . . and the concept of interacting with the leaflets with this technology is very familiar.
“It’s very important that things innovate,” he continued. “It'll be important for us to understand how this fits within the context of what we have now in terms of MitraClip. . . . Everybody will be very keen to see where this fits.”
Photo Credit: Praz F. Extracted from: Edwards PASCAL Mitral Valve Repair System. Mitral Valve Meeting 2017.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Praz F, Spargias K, Chrissoheris M, et al. Compassionate use of the PASCAL transcatheter mitral valve repair system for patients with severe mitral regurgitation: a multicentre, prospective, observational, first-in-man study. Lancet. 2017;390:773-780.
Alfieri O, Buzzatti N. Expansion of the treatment toolbox for mitral regurgitation. Lancet. 2017;390:722-724.
Disclosures
- Praz reports receiving personal fees from Edwards Lifesciences during the conduct of the study
- Sorajja reports receiving grant funding from Abbott Vascular.
- Alfieri and Buzzatti report no relevant conflicts of interest.


Jason Rogers