EARLY-AF: First-line Ablation Beats Meds for Recent Paroxysmal A-fib
The 300-patient trial is the largest to date, used implanted loop recorders to gauge A-fib burden, and saw no price paid for safety.
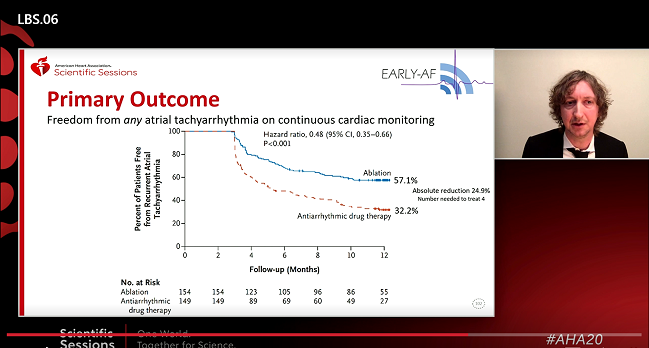
Using cryoablation as first-line therapy in patients with newly diagnosed, paroxysmal atrial fibrillation can significantly reduce the risk of recurrence measured by continuous arrhythmia monitoring over 1 year as compared with antiarrhythmic drugs, the EARLY-AF trial shows.
The benefit of ablation was also seen across a range of secondary endpoints, with no price paid in terms of adverse events, said Jason Andrade, MD (Vancouver General Hospital, Canada), who presented the results at the virtual American Heart Association 2020 Scientific Sessions.
“The major takeaway is that for every arrhythmia outcome we studied, ablation was significantly better than antiarrhythmic drugs,” Andrade told TCTMD. Taken along with STOP-AF First, which was presented at the recent European Society of Cardiology Congress, as well as earlier studies—RAAFT-1, RAAFT-2, and MANTRA-PAF—that used radiofrequency ablation, Andrade predicts an impact on guidelines, which currently recommend ablation as second-line therapy after drug treatment has failed.
“I think that the emergence of these studies will be enough to say we now have multiple randomized trials that are confirming the same finding: that ablation is more effective than drugs,” he said, “And you may see that the guidelines are more supportive, in select patients, of pursuing ablation as a first-line therapy.”
The EARLY-AF results were published simultaneously in the New England Journal of Medicine alongside STOP-AF FIRST.
EARLY-AF Results
The EARLY-AF trial enrolled 303 treatment-naive patients with newly diagnosed, symptomatic, paroxysmal A-fib who were randomized to first-line cryoablation or antiarrhythmic drugs. The study population was relatively young (average age 58), two-thirds male, and had symptomatic A-fib for a median of 1 year, reporting a median of three symptomatic episodes per month. All patients were also implanted with a loop recorder within 24 hours of ablation or initiation of drug therapy, with the device providing investigators with daily transmissions of A-fib episodes and burden. A 3-month run-in period allowed physicians to optimize patients on their medications, such that primary endpoint events were collected between day 91 and 1 year. Crossovers in the trial required independent committee review and approval. Strikingly for a drug-versus-ablation study where crossover rates have historically been in the range of 30%, Andrade noted, none occurred during the trial’s 1-year follow-up.
As Andrade showed today, A-fib recurrence rates at 12 months were 42.9% in the ablation group and 67.8% in the antiarrhythmic drug group (HR 0.48%; 95% CI 0.35-0.66), an absolute difference of 25%, yielding a number needed to treat of four.
Patients, he continued, are more concerned about symptomatic episodes of A-fib, but here, too, investigators saw a significant benefit with ablation. Freedom from symptomatic arrhythmia was 73.8% in the drug therapy group and 89% in the ablation group (HR 0.39; 95% CI 0.22-0.68), an absolute difference of 15%, with a number needed to treat of 6.5.
Atrial fibrillation burden, as measured by the loop recorder, was 3.3% lower in the ablation group. That “may or may not seem like a big number,” Andrade told TCTMD, “but I think if you contextualize it differently . . . 3% is 1 day out of every month of less AF.”
Quality-of-life improvements were seen in both groups at both 6 and 12 months, but the magnitude of benefit was greater—and by some metrics nearly double—in the ablated patients. Importantly, rates of serious adverse events and any safety events were low and no different between groups.
Adding in a Big Way
Discussing the results after Andrade’s presentation, Christine M Albert, MD, MPH (Cedars-Sinai Medical Center, Los Angeles, CA), called the study “very important” and “very well done,” then went on to list the specific trial attributes that “add in big ways” to prior evidence.
She singled out the trial’s size, making it the largest randomized study to date in this patient population; the use of implantable loop recorders, with recordings reviewed by blinded reviewers; aggressive medication titration with the goal of completely suppressing A-fib during the 3-month roll-in; the use of an independent committee to limit crossovers; and the use of multiple quality-of-life tools at different time points that clearly documented improvements.
Of note, said Albert, incidence of A-fib as measured by the loop recorders was “still pretty high” after ablation, even though symptomatic A-fib was much lower. “There’s a lot of potentially asymptomatic AF that is going on in these patients, another reason why we don’t stop anticoagulation at this point, but what really matters is symptomatic AF,” she said.
That said, Albert concluded, “EARLY-AF rigorously demonstrated the superiority of ablation over antiarrhythmic drugs for AF recurrence, quality of life, and AF burden as first-line therapy. Large-scale and longer-term studies are needed to determine whether these benefits might translate into improved cardiovascular outcomes.”
Mintu Turakhia, MD (Stanford University, CA), also commenting on EARLY-AF, pointed to the ways the field has moved forward this year alone.
“We know from EAST-AFNET4 that early rhythm control (drugs or ablation) led to a lower risk of cardiovascular events,” he said in an email. “So the question now isn't rate versus rhythm control, but rather, what should we be using for early rhythm control in patient likely to benefit? EARLY-AF demonstrated that in a predominantly paroxysmal AF population, early pulmonary vein isolation, compared to anti-arrhythmic drugs, led to a lower risk of recurrence, lower AF burden, and improved quality of life as measured by an AF-specific quality of life instrument. The caveat is that follow-up was only 1 year, but the study adds to the totality of evidence that early ablation is reasonable.”
Enough for the Guidelines?
To TCTMD, Andrade noted that investigators are planning to follow patients out to 3 years for the purposes of a cost-efficacy analysis, health resource utilization, as well as to gauge A-fib progression. Prior studies suggest that approximately 20% of patients treated with ablation have A-fib recurrence in the first 3 months, a number that falls to 10% in months 3 to 12 and then less than 5% per year thereafter. “If you're talking about super long-term time horizons, say 10 years, then that becomes a bit more of an evidence-free zone. We don't have many studies that have followed people that long,” he said. “In this study, there were 17 people in the ablation group who are re-ablated within the year, which is not inconsistent with ablation studies.”
Patients will also crossover from drugs to ablation during the follow-up period.
“As we get out to 3 years, you're probably going to see contamination in the sense that the drug patients will be crossing over to ablation as they fail, especially if we're seeing about a 50% failure rate within a year for drugs,” Andrade explained. “Then, for the ablation group, you'll probably have attrition in the long term where people are going to require multiple procedures. But the ideal is that you're getting at people early enough that they haven't developed enough abnormal atrial pathology to push them over the edge, whereas with more advanced atrial fibrillation like persistent A-fib, they're coming back for multiple procedures much more often, just because there’s so much more abnormality.”
Given the growing body of evidence, Andrade says he expects first-line ablation for paroxysmal A-fib to move up in the next iteration of the guidelines, although that may take some time. Current 2019 US guidelines and 2020 European guidelines give ablation a class I indication as a second-line option in patients with symptomatic atrial fibrillation refractory to or intolerant of antiarrhythmic drugs plus a class IIa indication as initial therapy for symptomatic paroxysmal A-fib.
Turakhia pointed out that it’s not a given that upfront ablation may have higher costs, noting: “In the US, class III drugs such as dofetilide require 2-3 nights of admission to the hospital for loading. How does that cost and value compare to AF ablation?”
He also stressed that risk factor reduction, and particularly blood pressure and weight control can’t be ignored. “It also is not carte blanche to overuse ablation in poorly selected patients,” said Turakhia. “Rather, this study, along with the totality of evidence—which also includes the concurrently published STOP-AF FIRST trial—shows that early ablation is a compelling and very safe strategy.”
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2020;Epub ahead of print.
Disclosures
- EARLY-AF was funded by a peer-reviewed grant from the Cardiac Arrhythmia Network of Canada with additional unrestricted grant support from Medtronic and Baylis Medical.
- Andrade reports grant support from Medtronic and Baylis, and personal fees from Biosense-Webster outside of the submitted work.
- Albert reports receiving an investigator-initiated grant from the NHLBI, St Jude, Roche Diagnostics, and Abbott.


Comments