EARLY TAVR Breaks New Ground for Preemptive Treatment of Asymptomatic AS
(UPDATED) While discussions about the lack of a mortality effect will continue, some say the findings may be enough to change guidelines.
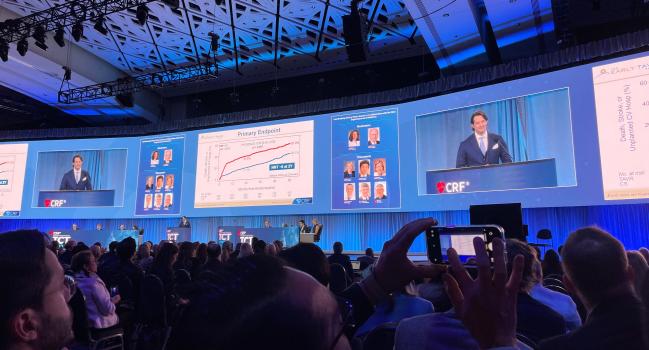
WASHINGTON, DC—Intervening early in patients with severe aortic stenosis (AS) but no symptoms may be more beneficial than watchful waiting, according to results from the EARLY TAVR trial.
At a median follow-up of 3.8 years, the primary endpoint of death, stroke, or unplanned CV hospitalization occurred in 35.1% of patients randomized to receive early TAVI versus 51.2% of those in a clinical-surveillance group (HR 0.50; 95% CI 0.40-0.63). That difference appeared to be driven by the unplanned hospitalizations, which included need for AVR in the first 6 months, that occurred in 41.7% of the surveillance patients and in 20.9% of those who had an early TAVI procedure.
Patients who had early intervention also were less likely to have worsening of left ventricular and left atrial function compared with those in the surveillance group.
“The most important finding is there's no evidence of any penalty or harm of the strategy of early intervention,” Philippe Généreux, MD (Morristown Medical Center, NJ), the trial’s principal investigator, told TCTMD.
Additionally, he said, the trial fosters a better understanding of the natural history of AS and when and how patients convert to symptoms.
“What we saw is 26% of the patients convert to symptoms within 6 months, 50% convert within a year, and more than 70% needed aortic valve replacement by 2 years,” Généreux noted. “About 40% of the patients convert with symptoms that are very advanced or acute, meaning pulmonary edema, New York Heart Association class III or IV heart failure, syncope, [or having] survived cardiac arrest. So, there's not a lot of reassurance in waiting.”
Généreux presented the findings here in the first late-breaking trials session of TCT 2024 and they were simultaneously published in the New England Journal of Medicine.
It seems that there's no advantages to wait. Philippe Généreux
Current guidelines recommend routine clinical surveillance every 6 to 12 months in patients with severe aortic stenosis and no symptoms. Two prior trials, RECOVERY and AVATAR, provided supportive evidence for early surgical AVR in severe aortic stenosis in the absence of symptoms.
In AVATAR, the primary composite outcome comprising all-cause death, acute MI, stroke, or unplanned hospitalization for heart failure was 15.2% in the early-surgery group and 34.7% in the conservative-management group at 32 months. In RECOVERY, which had 6-year follow-up, the composite of operative mortality or death from CV causes occurred in 1% of the early-surgery group and in 15% of the conservative-care group. At fewer than 160 patients in each of those trials, they were considerably smaller than EARLY TAVR and the patients were younger.
The three trials taken together, Généreux said, suggest to him that were someone to list pros and cons for early intervention versus surveillance in this patient population, there would be very few pros.
“It seems that there's no advantages to wait,” he said. “This is important, especially in light of the natural history of AS.”
Commenting for TCTMD, Josep Rodés-Cabau, MD, PhD (Laval University/Quebec Heart & Lung Institute, Canada), said that while the suggestion that early TAVI is a reasonable alternative to surveillance is a “very adequate conclusion” to draw from EARLY TAVR, he’s not fully convinced that’s the whole story.
“There are no differences in mortality at 4 years, which is an important point,” he said. “There also are no differences in stroke. The primary endpoint is positive, but it is [driven] mainly by patients who underwent the AVR later on.”
Rodés-Cabau noted that one in five patients in the early-intervention group experienced a CV event during follow-up even in the confines of a rigorously conducted trial. That lends support for the argument that the timeline for cardiac damage in asymptomatic severe AS remains uncertain.
“But, you could also argue that half of the patients in the clinical-surveillance group seemed to do okay,” he added. “In my opinion this tells us that the clinical surveillance that is recommended in the guidelines is probably not terrible, but definitely an earlier intervention seems to improve outcomes.”
Also commenting for TCTMD, Gilbert Tang, MD (Mount Sinai Health, New York, NY), said a key takeaway of EARLY TAVR is that asymptomatic severe AS is not a benign condition. “Even if you’re asymptomatic you should be monitored very closely for onset of symptoms or progression of insidious symptoms . . . so timely intervention can be done,” he said.
Tang pointed out that the EARLY TAVR cohort was overall a low-risk group on par with PARTNER 3. That’s important because over time, low-risk patients with symptomatic severe aortic stenosis in PARTNER 3 saw attenuation of differences in the primary composite endpoint, which had favored TAVI early on compared with surgery; the mortality curves for TAVI and surgery also crossed at 2 to 3 years.
The next few years should help sort out whether there are any long-term concerns about doing early TAVI in these patients, he added, particularly with regard to valve durability or concerns about the valve platform’s suitability for intervention.
The EARLY TAVR investigators have clinical and echocardiographic assessments planned for trial participants through 5 years.
Study Design and Results
EARLY TAVR enrolled 901 patients from 72 sites in the United States and three sites in Canada beginning in 2017. Of those, 455 were randomized to receive transfemoral TAVR (mean age 76 years; 29% women) with the Sapien 3 or Sapien 3 Ultra valve (Edwards Lifesciences) and 446 to a strategy of clinical surveillance (mean age 75.6 years; 33% women). The latter strategy involved standard management per American College of Cardiology and American Heart Association guidelines. If symptoms or other indications for aortic valve replacement developed those cases went before a review board, but the ultimate decision to do TAVI was made by the physician and the patient.
Patients had severe AS (defined as aortic valve area ≤ 1.0 cm2 or aortic valve area index ≤ 0.6 cm2/m2 with mean transaortic gradient ≥ 40 mm Hg or peak aortic jet velocity ≥ 4.0 m/s) and were classified as asymptomatic based on negative stress tests and detailed clinical history. In fewer than 10% of patients the classification was based on a detailed history alone due to physical limitations that prohibited a stress test. Included patients also were required to have a left ventricular ejection fraction of 50% or higher and an STS score of 10% or less. Approximately 84% of the cohort was classified as low risk by a heart team.
As noted, the primary composite endpoint favored earlier intervention, by an absolute difference of more than 16% at a median follow-up of 3.8 years, yielding a number needed to treat of six to prevent one event at 2 years.
All-cause mortality was 8.4% in the TAVI group and 9.2% in the surveillance group (HR 0.93; 0.60-1.44). Rates of stroke were 4.2% and 6.7%, respectively (HR 0.62; 0.35-1.10).
You could also argue that half of the patients in the clinical-surveillance group seemed to do okay. Josep Rodés-Cabau
The secondary outcomes included favorable outcome, defined as being alive at 2 years and having a Kansas City Cardiomyopathy Questionnaire (KCCQ) score ≥ 75 that did not decrease > 10 points from baseline. This endpoint favored TAVI at 86.6% versus 68.0% for surveillance patients (P < 0.001).
The composite of integrated left ventricular and left atrial function, defined as absolute left ventricular global longitudinal strain ≥ 15%, left ventricular mass index < 115 g/m2 for men or < 95 g/m2 for women, and left atrial volume index ≤ 34 mL/m2 occurred in 48.1% of the TAVI group and 35.9% of clinical surveillance patients (P = 0.001).
Mean KCCQ scores, which were 92.7 in both groups at baseline, were 94 in the TAVI group and 93 in the surveillance group at 2 years.
New-onset atrial fibrillation occurred in 13% of the TAVR group and 12.4% of the clinical-surveillance group (HR 1.08; 0.73-1.60), while the composite of death or disabling stroke occurred in 9.7% and 11.2% (HR 0.87; 0.58-1.31), respectively.
Among the 388 patients in the clinical-surveillance group who converted to AVR, the median time to conversion was 11 months and the median time from symptom onset or decision to intervene was 32 days. Approximately 2% converted with no symptoms. As of today, Généreux said, six patients remain in the surveillance group with no symptoms and no scheduled AVR.
“When they convert, they still convert with the same dramatic 40% ‘crash and burn’ scenario,” Généreux said in a press conference prior to the presentation. The real price of waiting for these long-term asymptomatic patients isn’t immediately clear.
“At 10 years or 6 years or 8 years, will they have more cardiac damage? Will they have more A-fib? The trend in increased stroke is real . . . and was very surprising to us,” he told TCTMD.
An exploratory analysis of the primary endpoint focusing on advanced signs and symptoms of heart failure was consistent with the main findings favoring early TAVR at 35.6% versus 55.4% for the surveillance group at 3.8 years (HR 0.49; 95% CI 0.40-0.62). In patients assigned to clinical surveillance, Kaplan-Meier estimates suggested greater risk of HF hospitalizations.
Guidelines and Next Steps
To TCTMD, Généreux said he believes the EARLY TAVR results will influence current guidelines regarding the timeline for management of asymptomatic severe aortic stenosis.
“It's always shocking to me when I read the mitral regurgitation guideline [because] even for patients with severe MR with no symptoms, as soon as there is, for example, atrial fibrillation, as soon as there is pulmonary hypertension, as soon as there is LV enlargement, it's an indication for mitral valve replacement,” he said. “In AS there's no such thing.”
Tang agreed that the EARLY TAVR data likely will have some impact on current recommendations.
“Perhaps there's some need for guidelines or consensus document changes to raise awareness of asymptomatic severe AS and the need to follow more closely. And more importantly, if [patients] are referred to the heart team for evaluation, even though they may not warrant treatment right away or the patient does not want it, it should be communicated clearly that a 3-month follow-up or [some other shorter time frame] is necessary given the findings of this trial rather than waiting for symptoms to happen or patients ending up hospitalized.”
Perhaps there's some need for guidelines or consensus document changes to raise awareness of asymptomatic severe AS and the need to follow more closely. Gilbert Tang
Rodés-Cabau said while the study will trigger significant debate, he doubts the guidelines will give a definitive nod to earlier intervention as the treatment of choice. “I would say it will be implemented in the guidelines as an alternative. Of that, I am convinced,” he added.
Another outstanding question is how the trial’s strict surveillance protocol and rapid timeline for treatment can be replicated in the real world given constraints on physicians and healthcare systems. Patients who converted to AVR were treated within a median of 32 days. Rodés-Cabau noted that unless the same rapid protocol is implemented, which may not be realistic in many countries and healthcare systems, the outcomes could prove very different, with those patients possibly paying an even greater price for waiting.
“The world has been waiting for the outcome of this trial,” discussant Bernard Prendergast, MD (St Thomas’ Hospital/Cleveland Clinic London, England), said following the presentation.
Addressing the question of whether the patients enrolled in EARLY TAVR are relevant to daily clinical practice, he said the answer “is an unequivocal yes.” Prendergast added that is now clear that patients require access to earlier diagnosis and they need to be prepared early for intervention.
“This will require education across referral networks to our general physician and general cardiology colleagues and I believe a mandated update to international guidelines with an early opportunity presented by the [European Society of Cardiology] update scheduled for next year.” Prendergast serves on the European Society of Cardiology/European Association for Cardio-Thoracic Surgery guideline-writing committee for the management of valvular heart disease.
Addressing a question from panelist Tsuyoshi Kaneko, MD (Washington University School of Medicine, St. Louis, MO), as to why AVATAR and RECOVERY—the early surgery trials—showed a benefit of early intervention on mortality while EARLY TAVR did not, Généreux reiterated that in addition to significant differences in patient characteristics, he believes the close surveillance in EARLY TAVR explains much of it.
Similar to Rodés-Cabau’s thoughts on the matter, he said such tight surveillance is unlikely to be practical, making the findings of lack of harm from early intervention all the more important to consider for real-world patients.
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Généreux P, Schwartz A, Oldemeyer B, et al. Transcatheter aortic valve replacement for asymptomatic severe aortic stenosis. N Eng J Med. 2024;Epub head of print.
Disclosures
- EARLY TAVR was sponsored by Edwards Lifesciences.
- Généreux reports consulting for and/or receiving speaking fees from Abbott Vascular, Abiomed, Boston Scientific, Caranx Medical, Cardiovascular System Inc, Edwards Lifesciences, GE Healthcare, iRhythm Technologies, Medtronic, OpSens, Pi-Cardiac, Puzzle Medical, Saranas, Shockwave, Siemens, Soundbite Medical Inc, Teleflex, and 4C Medical. He reports equity in Pi-Cardiac, Puzzle Medical, Saranas, and Soundbite Medical.
- Tang has received speaker's honoraria and served as a physician proctor, consultant, advisory board member, TAVR publications committee member, RESTORE study steering committee member, APOLLO trial screening committee member and IMPACT MR steering committee member for Medtronic, has received speaker's honoraria and served as a physician proctor, consultant, advisory board member and TRILUMINATE trial anatomic eligibility and publications committee member for Abbott Structural Heart, has served as an advisory board member for Boston Scientific and JenaValve, a consultant and physician screening committee member for Shockwave Medical, a consultant for NeoChord, Peija Medical, and Shenqi Medical Technology, and has received speaker's honoraria from Siemens Healthineers.
- Rodés-Cabau reports institutional research grants from and consulting for Edwards Lifesciences and Medtronic.






Comments