FDA Approves First Extravascular ICD
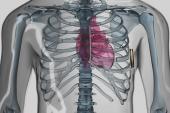
Implanted under the armpit with leads placed under the sternum, it avoids the potential for transvenous complications.

The US Food and Drug Administration has approved the Aurora Extravascular Implantable Cardioverter Defibrillator (EV-ICD; Medtronic) along with its Epsila EV defibrillation lead as an alternative to both traditional transvenous ICDs as well as other newer subcutaneous ICDs (S-ICDs), for the treatment of tachycardia and prevention of sudden cardiac arrest.
Use of the EV-ICD, which is implanted under the left armpit with leads placed under the sternum, avoids needing to have transvenous leads and the potential associated complications of vascular injury and vessel occlusion. The technology differs from the Emblem S-ICD (Boston Scientific), which also avoids transvenous lead placement but entails placing a lead above the sternum. The close proximity of the EV-ICD’s lead to the myocardium allows for antibradycardia and antitachycardia pacing that uses approximately half of the median energy that the S-ICD uses for defibrillation.
Approval of the Aurora EV-ICD was based on trial data published in the New England Journal of Medicine and presented at the Heart Rhythm 2023 meeting.
"The Aurora EV-ICD system is a tremendous step forward in implantable defibrillator technology," said study co-author Bradley P. Knight, MD (Northwestern Medicine Bluhm Cardiovascular Institute, Chicago, IL), in a press release. "Placing the leads outside of the heart, rather than inside the heart and veins, reduces the risk of long-term complications, ultimately allowing us to further evolve safe and effective ICD technology."
The Extravascular ICD Pivotal Study, with a total of 316 patients, showed that defibrillation therapy was successful in 98.7% of the 302 patients in whom ventricular arrhythmia could be induced, without any major intraprocedural complications or unique complications related to the procedure or system. At 6 months, 92.6% of patients were free from major system and/or procedure-related major complications such as hospitalization, system revision, or death. In all, 66 spontaneous arrhythmia episodes occurred in 16 patients, who received appropriate device response, including 18 discrete episodes converted to sinus rhythm.
By contrast, 29 patients in the study received 118 inappropriate shocks for 81 arrhythmic episodes during 10.6-months of follow-up, yielding a median number of inappropriate shocks of two per patient.
The press release notes that the Aurora device features longer battery life (11.7 years) and smaller size (33 cc) than other similar transvenous or subcutaneous ICDs.
Real-world performance and safety data are being collected for the Aurora system in the Enlighten global postapproval registry, which is expected to run for 5 years and enroll 1,000 patients.
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Medtronic. Medtronic receives FDA approval for extravascular defibrillator to treat abnormal heart rhythms, sudden cardiac arrest. Published and accessed on: October 23, 2023.



Comments