GUIDE-HF: Pulmonary Artery Sensor May Help in Mild HF, but COVID-19 Muddies Water
The trial missed its primary endpoint, but an analysis of the pre-COVID-19 period showed some signals of benefit.
(UPDATED) Muddy conclusions from the GUIDE-HF trial hint that pulmonary artery pressure monitoring with the CardioMEMS device (Abbott) may help prevent heart failure (HF) hospitalizations in a broader group of patients than previously shown, but the findings also expose the impact COVID-19 is having on getting clean, clear clinical trial results.
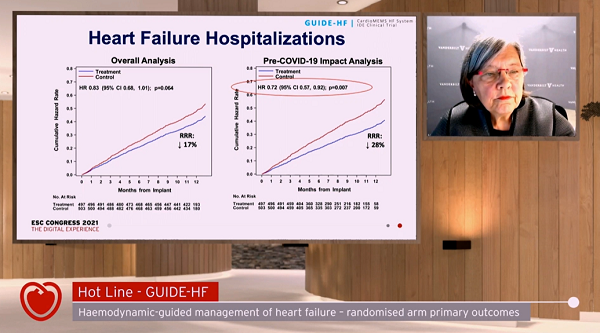
For the trial as a whole, no significant differences were seen in the primary endpoint in patients with NYHA II-IV heart failure randomized to hemodynamic monitoring as compared with patients managed by guideline-directed medical care alone. In the period prior to COVID-19 lockdowns, however, investigators saw a more marked drop in the number of patients dying or requiring hospitalization if their management could incorporate data provided by the CardioMEMS sensors.
“The primary endpoint of GUIDE-HF—reduction in heart failure events and mortality with hemodynamic-guided management—was not statistically significant in the overall analysis, but was statistically significant in the COVID-19 impact analysis,” JoAnn Lindenfeld, MD (Vanderbilt University, Nashville, TN), summarized during a press conference yesterday. The decision to take into account the pandemic’s inevitable disruptions to normal physician and hospital visits had been made in a blinded fashion after enrollment was complete and when just 3 months of follow-up had been collected.
“In this [pre-COVID-19] analysis,” said Lindenfeld, “the benefit of hemodynamic-guided management was primarily due to a 28% reduction in heart failure hospitalizations, the same reduction previously shown in the CHAMPION trial.”
CHAMPION was the study of NYHA class III patients with a prior HF hospitalization in the preceding 12 months. Positive results paved the way for regulatory approval of the CardioMEMS device in the United States in 2014, whereas CE Mark approval dates back to 2011 in Europe. GUIDE-HF was launched to see if the benefits of pulmonary artery pressure monitoring could extend to a wider patient group—those with NYHA class II or IV heart failure, or to patients with elevated natriuretic peptides but no prior hospitalization.
The device itself is a wireless, battery-less sensor implanted via a right heart catheterization into the pulmonary artery to remotely transmit pulmonary artery pressures that would warrant a change in management. Prior research, noted Lindenfeld, has established that increases in pressures typically precede hospitalizations by days or weeks, potentially allowing for up-titration of medications like diuretics that might stave off an urgent hospitalization.
Lindenfeld presented GUIDE-HF in the opening Hot Line session of the virtual European Society of Cardiology Congress 2021; the results were published simultaneously in the Lancet.
GUIDE-HF Results
In GUIDE-HF, 1,000 patients were implanted with the pulmonary artery pressure sensor between January and December 2019, then randomized to having their care informed by monitoring or to receiving standard-of-care management without access to sensor data. Patients, but not doctors, were blinded to study assignment.
At 12 months, 253 primary endpoint events had occurred in the 497 patients randomized to monitoring, and 289 in the 503 patients randomized to usual care (HR 0.88; 95% CI 0.74-1.05). Cumulative incidence of HF events (either hospitalizations or urgent HF hospital visits) also was not statistically different between groups. Hospitalizations alone were, again, numerically lower in the monitoring group, but the difference did not meet statistical significance.
COVID-19, however, emerged as having an independent effect on the primary endpoint, “warranting an analysis of all endpoints before the US national emergency declaration date of March 13, 2020,” the authors write.
During this early window, 177 primary events occurred in the sensor group and 224 events among controls (HR 0.81, 95% CI 0.66-1·00), a difference that was not maintained after the COVID-19 lockdowns were instituted, when event rates dropped markedly among the usual-care group but not in the monitored patients. Similarly, the cumulative incidence of heart failure events was significantly lower in the pre-COVID-19 impact analysis (HR 0.76, 95% CI 0.61-0.95). A total of 124 heart failure hospitalizations occurred in the sensor group and 176 in the control group, a 28% reduction mirroring what was seen in CHAMPION (HR 0.72, 95% CI 0.57-0.92).
To the media, Lindenfeld explained that too few NYHA IV patients were enrolled in the study, making it difficult to draw conclusions on the value of monitoring in patients with more severe impairment. But in patients with class II symptoms who had either a prior hospitalization for heart failure or elevated natriuretic peptides, monitoring appeared to be of benefit.
Moreover, the benefits of monitoring appeared consistent across a range of subgroups taking into account age, sex, race, heart failure etiology, and ejection fraction at study outset. Lindenfeld drew special attention to patients with no prior hospitalizations who appeared to benefit from the sensor, “providing a group of patients in whom early intervention is possible.” In addition, she said, “the results for heart failure with preserved EF are particularly important, as there are few approved therapies in this group.”
Asked about costs versus efficacy, Lindenfeld noted that such an analysis is planned but not yet completed; however, a cost-effectiveness analysis of CHAMPION suggested that the costs associated with the sensor are offset by savings driven by reduced HF events and hospital admissions.
Some Caveats
Commenting for TCTMD, Lynne Warner Stevenson, MD (Vanderbilt University Medical Center), noted that the benefits of pressure monitoring in GUIDE-HF, at least in the pre-COVID-19 analysis, “align with the totality of randomized and postapproval trials in the US and Europe, validating management guided by ambulatory cardiac filling pressures.” What’s added by this study, she said, is that this approach appears to also have an impact in patients with fewer symptoms and without prior hospitalizations.
What she perceives as the most “serious limitation” of the study, though, is the fact that patients were blinded—something insisted upon by regulatory agencies, not by the trial's steering committee. Patients were blinded not only to whether their sensor information was being used or not but, as a consequence, also to the fact that their physicians were acting on that information.
“This retro design prohibits the patient empowerment [that’s] vital to the emerging culture of self-health,” she said. “Our clinical experiences with these monitoring strategies reveal strong behavioral remodeling with patients in a close feedback loop and their enhanced sense of control over their heart failure. These benefits were withheld from subjects randomized in GUIDE-HF.”
More proof that pulmonary artery pressure monitoring helps is welcome, but not the greatest need in this space.
“We believers already believe,” she told TCTMD. What’s needed now are implementation studies in which both patients and physicians have access to sensor measurements, can learn from them, and can adjust therapies as needed. Indeed, she stressed, monitoring pulmonary artery pressures is about both keeping patients out of hospital and gaining a better understanding of how they respond to medication. She pointed to several studies documenting how some of the new HF medications affect pressures: information that would be empowering to both physicians with dosing strategies and polypharmacy choices, as well as patients in understanding their disease.
John G. F. Cleland, MD, and Pierpaolo Pellicori, MD (both University of Glasgow, Scotland), wrote the editorial accompanying GUIDE-HF’s publication. They point out that the trial may not have enrolled the “ideal” patient group, since mean pulmonary artery pressures at baseline were in the target range “with little possibility of short-term gain.” What’s more, the follow-up was likely too short to detect meaningful benefits of monitoring over time, and the data collected on medications suggests that while diuretics were more commonly changed in the monitored patients, these did little to substantially change pressures.
And that, they hint—echoing a point similar to that made by Stevenson—can only a starting point for understanding how best to use the information sensors provide in clinical practice.
“The GUIDE-HF results are encouraging but inconclusive, and should inform further research, possibly a large, simple, open-label trial to investigate a system of care rather than a single technology,” Cleland and Pellicori conclude.
In the discussion that followed the Hot Line presentation today, the designated discussant Frank Ruschitzka, MD (University Heart Center - Zurich, Switzerland), asked a series of rapid-fire questions, few of which Lindenfeld had time to answer, although she did clarify that diuretics were the most commonly used medication in response to sensor data. Of note, while most were up-titrations, one-third (a similar proportion to CHAMPION) were actually down-titrations. Over the course of the trial, the use of sodium-glucose cotransporter 2 (SGLT2) inhibitors increased from 1% to about 8% in both groups, she added.
Rory Collins, FMedSci (University of Oxford, England), one of the session panelists, offered some of the sharpest criticism of the trial, questioning the P value used to justify the COVID-19 analyses. “Irrespective of what the FDA has said, a P value of 0.15 is not a good test for heterogeneity between the pre- and post-COVID period, and wasn’t the threshold used when considering the other subgroups where the interpretation is that there isn’t any significant, material heterogeneity,” he argued. “I accept the point that potentially this intervention is effective, but I think one has to put the greatest emphasis on the overall result.”
Lindenfeld, in response, said they could “argue about” the use of the 0.15 cut point but, in her mind, the analysis was justified. “What we saw during COVID is that all of a sudden, everywhere, heart failure hospitalizations dropped. In our own experience at Vanderbilt, they were no sicker when they came in, but there were fewer patients coming in. . . . I think we all agree, just practically, that there was an interaction with COVID with everything that we do, and that’s why all the regulatory agencies and professional societies recommended a change in the statistical analysis plan to account for that.”
Also commenting, Lancet Senior Executive Editor Stuart Spencer, PhD, was more enthusiastic about the trial saying, “I’m actually pleased that it is effectively positive, mirroring what we saw in CHAMPION; it just didn’t achieve statistical significance. So I think that there’s something there.” He continued: “What we have here is a trial that tells us about physiology but not necessarily, at least in my view, . . . a real treatment that is going to be rolled out around the world or even in the US and Canada.”
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Lindenfeld J, Zile MR, Desai AS, et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet. 2021;Epub ahead of print.
Cleland JGF, Pellicori P. To master heart failure, first master congestion. Lancet. 2021;Epub ahead of print.
Disclosures
- The study was funded by Abbott.
- Lindenfeld reports research grants from AstraZeneca, Sensible Medical, and Volumetrix and is a consultant for Abbott, Alleviant Medical, AstraZeneca, Boehringer Ingelheim, Boston Scientific, CVRx, Edwards, Impulse Dynamics, and VWave.
- Stevenson reports having no financial conflicts with industry, but that she does provide unpaid consultation to Abbott and other companies regarding heart failure management.
- Cleland reports personal fees from Abbott for serving on the advisory board for the MitraClip device unrelated the CardioMEMS device.
- Pellicori reports no conflicts of interest.




Comments