MIDAS Touch: Anatomic Strategy Dramatically Lowers Pacemaker Rate With Evolut Valves
The high PPM rates with self-expandable valves can be mitigated when TAVR is guided by patient anatomy, say researchers.
A patient-specific strategy focused on device positioning during TAVR procedures with a repositionable, self-expandable transcatheter heart valve (Evolut R/PRO; Medtronic) leads to significant decreases in permanent pacemaker implantation and left bundle branch block, according to the results of a new study.
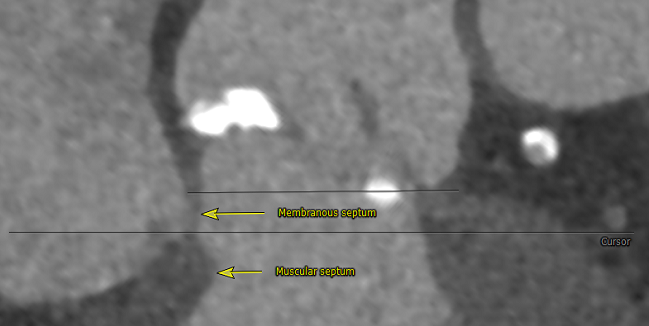
By measuring the length of the membranous septum (MS) during preprocedural CT and then targeting a valve implantation less than the MS length, operators lowered the rate of permanent pacemaker implantation to 3.0% and new-onset left bundle branch block to 9.0%. These rates are considerably lower than those achieved at their center prior to initiating this individualized strategy and are significantly lower than those observed in clinical trials.
With this patient-specific, anatomy-based approach, investigators hope they can level the playing field for operators treating patients with repositionable, self-expanding transcatheter heart valves.
“An important goal for TAVR is to standardize outcomes and to make it less operator dependent and more consistent internationally,” lead investigator Hasan Jilaihawi, MD (NYU Langone Medical Center, New York, NY), told TCTMD. While experienced, high-volume institutions have reported pacemaker rates below those observed in the clinical trials, which are as high as 25% with the self-expandable transcatheter heart valves, Jilaihawi hopes their strategy will lower the pacemaker rate at all TAVR centers.
It’s a simple concept of bespoke therapy, tailoring the approach to the patient’s anatomy. Hasan Jilaihawi
“With TAVR we now have very low rates of stroke and death that have really impacted the type of populations we’re treating,” he said. “We’re now treating low-risk populations, and it’s a big deal in a low-risk patient to live with a pacemaker. They’re younger and will have to live with a pacemaker for longer. It is an important complication to avoid, and we ideally want to achieve rates at least as low as surgery.”
The findings, which Jilaihawi described as “dramatic,” still need to be validated in a larger multicenter series, but Josep Rodés-Cabau, MD (Laval University/Quebec Heart and Lung Institute, Canada), who wrote an editorial accompanying the study, says he is impressed by the advance in the “battle against conduction disturbances post-TAVR.” The study, he writes, shows that applying a meticulous valve implantation technique, one that tries to avoid mechanical interference with the His bundle which passes through the MS, “resulted in one of the best results ever reported regarding the occurrence of conduction disturbances and permanent pacemaker implantation post-TAVR.”
Pacemaker Rates Remain Troublesome
Conduction disturbances, particularly the need for permanent pacemakers, have been the Achilles’ heel for TAVR when compared with the surgical approach, a problem that has afflicted the self-expanding devices more than the balloon-expandable transcatheter heart valves. In the first study of patients at extreme risk for surgery, the permanent pacemaker rate with CoreValve (Medtronic) was more than 25%. The rate remained high in the intermediate-risk trial, and while it declined in the most recent trial testing TAVR with CoreValve, Evolut R, and Evolut PRO versus surgery in low-risk patients, it remained a concern, at 17.4%.
In this retrospective study of 248 patients with severe aortic stenosis undergoing TAVR, which was published August 28, 2019, in JACC: Cardiovascular Interventions, the researchers first sought to identify anatomical, electrophysiological, and procedural factors that were associated with the need for permanent pacemaker. All patients were treated with contemporary self-expanding TAVR devices from Medtronic, including the larger Evolut R 34 XL approved for larger annular diameters, via transfemoral access under conscious sedation.
In the prospective series, operators implemented the anatomically guided implantation strategy in 100 patients with severe aortic stenosis, which they labeled the Minimizing Depth According to the Membranous Septum, or the MIDAS approach. Specifically, operators positioned the device at a prerelease depth less than the MS length on the preprocedural CT. In doing so, the rate of permanent pacemaker implantation at 30 days was 3.0%, which was significantly lower than the rate in the retrospective series (P = 0.035). Similarly, the rate of new left bundle branch block declined from 25.8% in the retrospective series to 9.0% for patients treated with the MIDAS strategy.
Landing Device Above Membranous Septum
To TCTMD, Jilaihawi said operators aim to “land” the device as high as possible (less ventricular depth) but use imaging to fine-tune their position and recapture the device if it initially lands deeper than the MS. In the prospective series using the MIDAS strategy, the mean implant depth was 2.3 mm. The implant depth was only 1.0 mm less than the implant depth using a standard TAVR approach, but the proportion of patients with an implant depth smaller than the MS length was considerably greater (79.8% vs 54.8%).
“If the device is implanted too deep, deeper than the membranous septum length, it can press directly on the conduction system, which can lead to left bundle branch block or heart block requiring a pacemaker,” said Jilaihawi. “My partners and I will not do a CoreValve procedure now without knowing the membranous septum length,” he added. “We’re one of the busiest CoreValve centers in the world, and [the MS measurement] has become an indispensable measurement when we’re doing TAVR.”
The researchers have continued to use the individualized MIDAS strategy in more than 300 patients at their center, where the rate of pacemaker implantation remains under 5%. “It’s a real finding,” he said. “Our pacemaker rate with CoreValve is very close to the rate with surgical valves.” For patients undergoing surgical aortic valve replacement, the pacemaker rate typically ranges from 4.0 to 6.0%, the investigators note.
The bottom line from their new report, said Jilaihawi, is that the pacemaker rate is more dramatically modifiable when the implantation is guided by patient anatomy. “We’re using the imaging to guide the procedure, and in doing so, we’re able to improve the outcomes with a repositionable device,” he explained. “It’s a simple concept of bespoke therapy, tailoring the approach to the patient’s anatomy.”
In his editorial, Rodés-Cabau notes that the levels of intra- and interobserver variability for MS measurements were very good, but it is unknown if such consistent measurements can be obtained in other clinical centers. He questions how often valves needed to be recaptured and repositioned during TAVR, noting that this could have a negative impact on cerebral emboli events. He also expresses worries about potential risks of valve dislocation/embolization when aiming for a high implant depth, particularly when the strategy is expanded to other operators and hospitals.
Despite these questions, Rodés-Cabau says obtaining “single digit” rates of pacemaker implantation and new-onset left bundle branch block is “of high clinical relevance in the era when TAVR is going to expand to the treatment of low-risk and younger patients.” These latest findings, he concludes, “represent an important advance in this direction and indicate that that the battle against conduction disturbances post-TAVR can be won.”
Photo Credit: Hasan Jilaihawi, MD
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Jilaihawi H, Zhao Z, Du R, et al. Minimizing permanent pacemaker following repositionable self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol Intv. 2019;Epub ahead of print.
Rodés-Cabau J. Optimizing valve implantation depth to win the battle against conduction disturbances post-TAVR. J Am Coll Cardiol Intv. 2019;Epub ahead of print.
Disclosures
- Jilaihawi reports consulting to Edwards Lifesciences and Venus Medtech; he reports research grants and research support from Medtronic and Abbott Vascular.
- Rodés-Cabau reports institutional research grants from/consulting for Edwards Lifesciences, Medtronic, and Boston Scientific.


Comments