More Registry Data Show Post-TAVI Benefits With Sentinel but No Stroke Impact
Some argue new trial designs or devices are needed in this space; others say using cerebral embolic protection is “wishful thinking.”

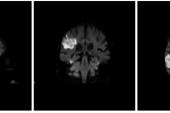
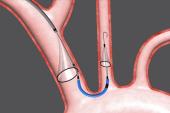
WASHINGTON, DC—Cerebral embolic protection (CEP) with the Sentinel device (Boston Scientific) reduces the number and volume of new lesions identified by brain MRI immediately following TAVI, but this does not seem to affect short-term clinical outcomes, according to new Korean registry data.
With no definitive randomized data supporting a significant effect of CEP on reducing stroke in TAVI patients, practice has varied according to operator preference and institutional availability. PROTECTED TAVR, the largest trial in this space, failed to show an overall effect of embolic protection on strokes but did demonstrate safety and a reduction in disabling stroke, though the study was not powered for that endpoint.
“In real-world practice, the protective effect and clinical outcomes of protection devices remains controversial,” said Han-Su Park, MD (Asan Medical Center, Seoul, Republic of Korea), who presented his findings yesterday at TCT 2024. As such, “it is necessary to find the relationship between the improved brain MRI imaging and clinical outcomes such as peripheral procedural stroke or neurocognitive dysfunction.”
The positive secondary endpoint of PROTECTED TAVR coupled with many neurologists arguing that the lesions seen on diffusion weighted MRI are strokes have “led to continued support but no reimbursement in the United States for the Sentinel device,” said Gregory Fontana, MD (HCA Los Robles Regional Medical Center, Thousand Oaks, CA), who discussed the study following Park’s presentation. However, it remains open to interpretation whether the new findings confirm the existing data and if CEP is still needed going forward, he said.
Commenting to TCTMD, Samir Kapadia, MD (Cleveland Clinic, OH), the primary investigator of PROTECTED TAVR, called the findings “exciting” and said he expects they will “help the field because there [have been] many setbacks with controversial data on CEP.”
However, not everyone is as positive. John Carroll, MD (University of Colorado School of Medicine Anschutz Medical Campus, Aurora), told TCTMD in an email that according to unpublished TVT Registry data, CEP use in the United States has “fallen year by year” since PROTECTED TAVR was published. This is “despite the manufacturer’s marketing claims suggesting the trial was positive,” he noted.
Further, he continued, “Sentinel is a costly technology, prolongs the procedure, and has failed to lower the risk of stroke during TAVR, despite our hopes based on debris capture.”
Registry Findings
For the study, Park and colleagues looked at 35 consecutive patients with severe aortic stenosis who underwent transfemoral TAVI with CEP at their institution between May 2022 and September 2023. They used a control group of 160 similar patients undergoing TAVI without CEP but with corresponding brain MRI data from the ADAPT-TAVR trial. All patients received brain MRI scans at baseline before their TAVI, within 2-7 days of their procedure, and again at 6-month follow-up.
Mean patient age was 80.4 years in each cohort, but CEP-treated patients were more often male (62.9% vs 41.9%; P = 0.024) and more apt to have histories of carotid stenosis (20% vs 5%; P = 0.007) and lung disease (8.6% vs 31.3%; P = 0.006). Medication use, too, differed between the groups, with the Sentinel population more likely to be prescribed dual antiplatelet therapy (91.4% vs 48.8%) and less likely to be prescribed non-vitamin K antagonist oral anticoagulants (8.6% vs 51.3%; P < 0.001).
Contrast volume use in TAVI with CEP was higher than that in controls (mean 263.24 vs 231.7 mL; P = 0.026) and device success was significantly lower (97.1% vs 100%; P = 0.024), but procedure times were similar (mean 67.7 vs 74.0 minutes; P = NS). All Sentinel procedures were performed radially with balloon-expandable valves and a 25.7-minute mean device-deployment time. Success of both device deployment and retrieval were 100%.
The co-primary endpoints of the number (2 vs 9) and volume (223.2 vs 450 mm3) of new cerebral lesions on brain MRI between baseline and immediately post-TAVI were significantly lower in the Sentinel group compared with controls. When the lesions were stratified by protected, partially protected, and nonprotected, the protected-area lesions decreased significantly in both number (1 vs 6) and volume (120.2 vs 289.2 mm3) and partially protected lesions decreased significantly by number (1 vs 4; P < 0.001 for all).
At 6 months, however, there were no longer any differences in new lesion number (1 vs 1) or volume (58.6 vs 69.8 mm3) between the Sentinel and control arms. At 1 month, the clinical outcomes of all-cause death, stroke, MI, permanent pacemaker, acute kidney injury, bleeding, and rehospitalization were similar between the groups.
More Research Needed?
Kapadia pointed out a key difference with this study is that all patients were treated with balloon-expandable valves while in PROTECTED TAVR 25% of devices were self-expanding. “Balloon expandable valves have almost better reduction in all strokes, [although] not that different for major, compared to self-expanding valves with Sentinel,” he explained in an email.
Notably, Kapadia said, the lesion volume seen with Sentinel here is similar to that in the Sentinel IDE trial but higher with controls, potentially indicating a “higher-risk population with more bicuspid or calcified valves.”
This study is also subject to a limitation that was seen with PROTECT TAVR: that many patients cannot undergo a postprocedural MRI. “It may be important to know why the patients could not have MRI post-TAVR in Sentinel group,” he suggested.
Going forward, Kapadia said he would like to see more research in this space. While a superiority trial looking at clinical endpoints between Sentinel and controls “is impossible because of the sample size needed,” he said new devices might be studied in a superiority analysis with Sentinel. “Since there are a lot of data on clinical outcomes with Sentinel (and also MRI data to compare), it may be possible to extrapolate clinical benefit if the devices are compared to Sentinel with an MRI endpoint,” he said.
During the session, panelist Victor Alfonso Jimenez Diaz, MD (Hospital Alvaro Cunqueiro, University Hospital of Vigo, Spain), agreed on the need for new devices and approaches to definitively decrease the rate of post-TAVI stroke. “Even using Sentinel, we're still seeing some small cerebral insults in the brain, and this is important as we are going to treat younger patients,” he said. “Potentially, we're missing this primary endpoint because we have to look at a really long-term outcome. And for that, in order to achieve a better result, potentially we need to use different devices that provide complete cerebral protection.”
The challenge here may be with the endpoint of stroke to begin with, according to panelist Ariel Finkelstein, MD (Tel Aviv Medical Center, Israel). “How many times has it happened to you that the wife of a patient came to you 1 month after and told you he’s not the same?” he asked the panel, acknowledging that this experience isn’t a measurable endpoint. “But it happens. There's no chance that again and again we're having more particles, more lesions, more everything and no more strokes. So something is wrong.
“I've heard again and again that there's nothing worse than stroke,” Finkelstein continued. “I think it's about time we change the algorithm to not look for the solid endpoint of stroke, but rather something else.”
Agreeing on a metric with which to measure neurocognitive decline in these patients will prove challenging, he added.
Panelist Pilar Jimenez-Quevedo, MD, PhD (Hospital Universitario Clinico San Carlos, Madrid, Spain), suggested that a change in trial design might help. “We need a trial including only patients at high risk of a stroke, such as valve-in-valve, bicuspid, or severely calcified valves,” she said. “The incidence of stroke is very low, so you need to include only patients at high risk to get a significant difference in clinical results.”
There has been enough time to determine the best endpoint to prove that Sentinel would work, argued panelist Vinicius Esteves, MD, PhD (Rede D'Or São Luiz, São Paulo, Brazil). “Honestly, I don't know if it's a problem in the design of the trial or if it's actually the device,” he said. “Something needs to be changed. . . . I think we need to improve the device to get better clinical outcomes.”
On the other side, Carroll argued it may be time to move on. “There continues to be a disconnect, on many levels, between presumed pathophysiology of embolic strokes, capturing debris, MRI lesions, and prevention of clinical events,” he said. “Until we understand this field better and until strong evidence emerges of clinical benefit, the use of CEP should remain limited, at the local operator’s discretion (unless administratively restricted), and grounded in wishful thinking, not science.”
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Park H-S. Short- and long-term effect of cerebral embolic protection device on new brain lesion following transcatheter aortic valve replacement: the cohort study of Sentinel registry (SENTINEL). Presented at: TCT 2024. October 27, 2024. Washington, DC.
Disclosures
- This study was partly supported by the CardioVascular Research Foundation (CVRF) and Boston Scientific.
- Park and Carroll report no relevant conflicts of interest.
- Kapadia reports being the primary investigator for the PROTECTED TAVR trial, which was funded by Boston Scientific.




Comments