Novel Interatrial Shunt Succeeds in HF Hemodynamic Study: REDUCE LAP-HF I
The study sets the stage for larger trials and offers hope for patients with HFpEF, who have limited treatment options, say researchers.
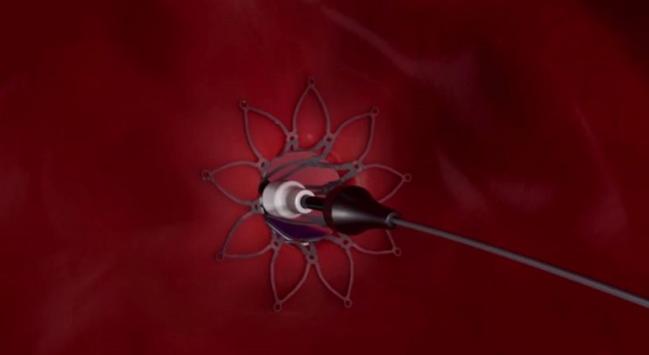
ANAHEIM, CA—An intracardiac shunt device safely unloads pressure within the left atrium among patients with heart failure and preserved or mildly impaired left ventricular function, according to the results of a new study.
In the randomized, sham-controlled, clinical trial, the novel shunt, which is delivered via femoral venous access and requires a transseptal puncture, decreased pulmonary capillary wedge pressure (PCWP) during exercise in patients with heart failure with preserved ejection fraction (HFpEF) or with modestly impaired ejection fraction.
The results of the mechanistic REDUCE LAP-HF I, which were presented here last week at the American Heart Association (AHA) 2017 Scientific Sessions by Sanjiv Shah, MD (Northwestern University Feinberg School of Medicine, Chicago, IL), were published simultaneously online in Circulation. The randomized, phase II study builds on the findings of REDUCE-LAP, a single-arm safety study released at AHA 2016, setting the stage for larger clinical trials and offering hope for the treatment of patients in which therapeutic options are limited, say researchers.
“There is no clear medical therapy for HFpEF,” lead investigator Ted Feldman, MD (NorthShore University Health System, Evanston, IL), told TCTMD. “Medical therapy today is effectively treating hypertension and whatever associated conditions there might be.”
The HFpEF population is roughly equal in size to the number of patients with HF and reduced ejection fraction (HFrEF) and the prognosis is nearly identical in both subsets. “It’s a large population with an unmet need,” said Feldman. “The prognosis for HFpEF, if you look at survival curves, is similar to HFrEF, with most studies that have compared them showing the survival curves to be indistinguishable or to have very little difference.”
HFpEF Population Growing
To TCTMD, Feldman explained that patients with HFpEF are known to have impaired left ventricle relaxation and compliance. In these small, stiff left ventricles, any pressure overload is transmitted to the left atrium and then to the lungs. This results in an increase in pulmonary venous pressures, particularly during exercise, and symptoms of dyspnea and exercise intolerance.
“The concept of the interatrial shunt is to allow decompression of the left atrium,” said Feldman. The device, he added, is not unlike an atrial septal defect occluder, except it has a hole in the middle that allows the left atrium to offset the pressure overload.
In the study, investigators randomized 44 symptomatic patients with preserved or mildly reduced heart failure (EF ≥ 40%) despite optimal medical therapy to treatment with the interatrial shunt (Corvia Medical) or to a sham procedure. In the control arm, patients underwent intracardiac or transesophageal echocardiography of the atrial septum and left atrial appendage but did not receive the transseptal puncture.
Treatment with the interatrial shunt resulted in a larger reduction in PCWP—a surrogate marker of left atrial pressure—during exercise at 1 month when compared with the control group. In terms of secondary endpoints, the change in peak PWCP at 1 month was -3.5 mm Hg in the treatment group compared with -0.5 mm Hg in the control arm, a nonsignificant difference. Exercise time increased by 1.2 minutes with the shunt and 0.4 minutes in the control arm (P = 0.60).
The present study, said Feldman, was meant to show how the shunt works, which is via the reduction in PCWP during exercise. The researchers have recently begun enrolling patients in REDUCE LAP-HF II, a blinded, randomized, clinical trial testing the interatrial shunt in 380 patients with HFpEF (EF ≥ 40%). In that pivotal trial, the primary endpoint includes clinical outcomes, quality-of-life assessments, and changes in 6-minute walk distance.
“What we believe we established pretty clearly is that if patients have an improvement in symptoms and walk time in the pivotal trial, that improvement can be attributed to the mechanism of the device,” said Feldman. If successful, this “really could be a major milestone in heart failure therapy,” he added.
Lynne Stevenson, MD (Brigham and Women’s Hospital, Boston, MA), who commented on the study following the AHA presentation, called the interatrial shunt an “ingenious strategy” for coping with a condition that has little recourse aside from the use of diuretics to deal with fluid retention and hypertensive medications. Use of the device, she noted, addresses both the symptoms of heart failure and progression of the disease.
That said, Stevenson pointed out that HFpEF is a heterogeneous phenotype and includes patients with and without obesity, those with and without clinical evidence of elevated cardiac filling pressures at rest, and those with and without renal disease. Additionally, HFpEF includes patients with different degrees of right ventricular dysfunction. Future studies, she said, will need to address the effects of the shunt in such patients, as well as to address the long-term impact of uploading the right atrium on right ventricular function.
Photo Credit: Image screen capture courtesy of the Corvia Medical website.
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Feldman T, Mauri L, Kahwash R, et al. A transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP-HF I): a phase 2, randomized, sham-controlled trial. Circulation. 2017;Epub ahead of print.
Disclosures
- The study was funded by Corvia Medical.
- Feldman reports receiving consulting fees from Abbott, Boston Scientific, Edwards Lifesciences, and Gore.
- Shah reports receiving research grants from Actelion, AstraZeneca, Corvia, and Novartis and consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cardiora, Eisai, Ironwood, Merck, Novartis, Sanofi, and United Therapeutics.


Comments