PARADISE-MI: ARNI Doesn’t Surpass ACE Inhibitor After Acute MI
Despite the missed endpoint, sacubitril/valsartan was safe and supports an incremental clinical benefit, researchers conclude.
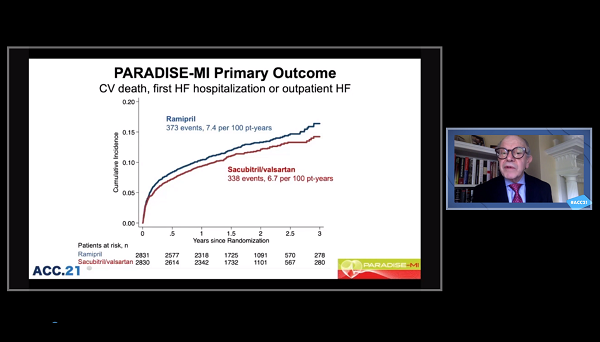
For immediate post-MI patients, sacubitril/valsartan (Entresto; Novartis) does not significantly decrease the risk of heart failure (HF) or CV death compared with an ACE inhibitor, the PARADISE-MI trial shows.
“Our prespecified observations of reductions in both the composite for CV total events, as well as investigator-reported events, support an incremental clinical benefit of sacubitril/valsartan,” said lead investigator Marc A. Pfeffer, MD, PhD (Harvard Medical School and Brigham and Women’s Hospital, Boston, MA), in his late-breaking presentation today at the virtual American College of Cardiology 2021 Scientific Session. “The safety and tolerability of sacubitril/valsartan in this acute MI population without a run-in was comparable to that of a proven, well-used ACE inhibitor.”
But the disappointing results mean physicians now have more questions as to whether this drug might have a niche in a broader patient group, and whether that incremental benefit has clinical value, particularly given the drug’s already-low uptake.
The safety and tolerability of sacubitril/valsartan in this acute MI population without a run-in was comparable to that of a proven, well-used ACE inhibitor. Marc Pfeffer
“Regardless of P values and percents, obviously the per-patient effect is what we care most about, and regardless of how you combine the endpoints, it's really not going to be more than about one event per 100 patient-years, which is, of course, small,” noted panelist Lynne Warner Stevenson, MD (Vanderbilt University Medical Center, Nashville, TN), following Pfeffer’s presentation. Although sacubitril/valsartan is not approved for use in patients who do not yet have HF, she asked him: “If you had ARNI and ACE in your pocket for 50 patients each and you had 100 of your PARADISE-type patients, who would you give the ARNI to?”
Pfeffer said while PARADISE-MI can’t answer that question at this time, the positive safety data should allow researchers to “take a deep breath and take a deep dive” in search of patient subgroups who may benefit most from sacubitril/valsartan in this clinical scenario.
“What subgroups you do have suggest it's the sicker patients who benefit the most,” Stevenson added. “Perhaps it's because those who are destined to go on to heart failure are actually just beginning their heart failure treatment early.”
Pfeffer said the focus going forward will be on probing the time sequence. “We're going to be producing some landmark analyses, which I think will let us speak to this. It might mean that earlier use of sacubitril/valsartan, rather than waiting for severe symptoms or waiting for symptoms, may help. But I can't say that at this moment,” he added.
Investigator-Reported Outcomes Show Advantage
For PARADISE-MI, Pfeffer and colleagues enrolled 5,669 patients from 41 countries who had had an acute MI within the previous 7 days (mean 4.3 days). None had HF, but all had transient pulmonary congestion and/or LVEF ≤ 40%, plus at least one additional risk factor for HF or death: age ≥ 70 years, estimated glomerular filtration rate < 60 mL/min/1.73m2, diabetes, prior MI, atrial fibrillation, LVEF < 30%, Killip class ≥ III, or STEMI without reperfusion. In each group, 92% were on dual antiplatelet therapy, 85% were on a beta-blocker, and 78% were on an ACE inhibitor or ARB.
Patients were randomized to ramipril (target dose of 5 mg BID) or sacubitril/valsartan (target dose of 97/103 mg BID), with three matching blinded dose titrations.
At a follow-up of 23 months, the combined endpoint of CV death, first HF hospitalization, or development of outpatient HF had occurred in 11.9% of the sacubitril/valsartan group and 13.9% of the ramipril group (HR 0.90; 95% CI 0.78-1.04). That translated to 7.4 events per 100 patient-years for ramipril and 6.7 events per 100 patient-years for sacubitril/valsartan.
The components of the primary outcome all showed trends toward lower numbers of events with sacubitril/valsartan versus ramipril, but those also were not statistically significant. Among the 23 prespecified subgroups, only two—patients age ≥ 65 and those who received PCI—showed a trend toward greater benefit with sacubitril/valsartan than ramipril.
For all secondary endpoints, the comparisons favored sacubitril/valsartan: CV death or HF hospitalization (HR 0.91; 95% CI 0.78-1.07); HF hospitalization or outpatient development of HF (HR 0.84; 95% CI 0.70-1.02); CV death, nonfatal MI, or nonfatal stroke (HR 0.90; 95% CI 0.77-1.05); CV death and total hospitalizations for HF, MI, or stroke (RR 0.84; 95% CI 0.70-1.00); and all-cause death (HR 0.88; 95% CI 0.73-1.05).
In exploratory analysis looking at total events, however, the difference between ramipril (n = 539) and sacubitril/valsartan (n = 452) did reach statistical significance for reduction in events (RR 0.79; 95% CI 0.65-0.97). Similarly, investigator-reported outcomes showed an advantage for sacubitril/valsartan over ramipril for the primary endpoint (HR 0.85; 95% CI 0.75-0.96), as well as for development of outpatient HF (HR 0.69; 95% CI 0.54-0.88).
Looking at adverse events, the sacubitril/valsartan group had more hypotension than did the ramipril group (28.4% vs 22.0%) but fewer reports of cough (9.0% vs 13.1%) or liver abnormalities (4.7% vs 5.9%; P < 0.05 for all comparisons). Drug discontinuation was similar in both groups, with fewer discontinuations for cough or hypotension in the sacubitril/valsartan group.
During the trial, 242 people died in the ramipril arm and 213 died in the sacubitril/valsartan arm (P = 0.16).
Reservoir of MI Survivors at Risk Increasing
Pfeffer pointed out that the contemporary HF pharmacology has resulted in dramatic reductions in mortality rates, first with ACE inhibitors in the 1990s, then with valsartan in the early 2000s, and finally with sacubitril/valsartan today. However, he cautioned that HF investigators need to continue to pursue the goal of further decreases, particularly given the growing population. “We have more and more people surviving an infarct, so that reservoir of people who develop heart failure is increasing,” he said.
Mary Norine Walsh, MD (St. Vincent Heart Center, Indianapolis, IN), who spoke in a press conference with reporters after the presentation, said while PARADISE-MI provides “exceedingly reassuring data” on the safe use of sacubitril/valsartan, getting the drug to patients with HF who qualify for it is already complicated.
“The prior authorization process is unbelievable, and for some of our patients, they have no access unless they pay fully for the drug,” she said. “So, that's an important real-world problem that we face with this extraordinary therapy.”
L.A. McKeown is a Senior Medical Journalist for TCTMD, the Section Editor of CV Team Forum, and Senior Medical…
Read Full BioSources
Pfeffer M. Prospective ARNI versus ACE inhibitor trial to determine superiority in reducing heart failure events after myocardial infarction (PARADISE-MI). Presented at: ACC 2021. May 15, 2021.
Disclosures
- PARADISE-MI was sponsored by Novartis.
- Pfeffer reports consulting fees/honoraria from AstraZeneca, Boehringer Ingelheim-Lilly, Corvidia, DalCor, GlaxoSmithKline, Novartis, Novo Nordisk, Peerbridge, and Sanofi-Aventis; equity in DalCor; and research/research grants to his institution from Novartis.





Comments